Nanotheater
Collaborative research projects have provided us with opportunities to study interesting materials and surface processes. This page highlights a few examples of team efforts for studies with scanning probe microscopy.
Nanoscale Studies of the Corrosion of Copper Surfaces in Water (USEPA)
The mechanisms of surface passivation and the evolution of nanoscale mineral deposits on surfaces at very early stages of the corrosion of copper surfaces in water was investigated using AFM. Orthophosphate and pH dramatically influence the evolution and progression of changes during surface corrosion. In collaborative studies with the USEPA research laboratory in Cincinnati, we designed AFM experiments to provide insight into the mechanisms of surface passivation and the evolution of nanoscale mineral deposits on copper surfaces at very early stages of surface corrosion in water. [Lewandowski, B. R.; Lytle, D. A.; Garno, J. C. Nanoscale investigation of the impact of pH and orthophosphates on the corrosion of copper surfaces in Water. Langmuir, 2010, 26(18), 14671-14679.]
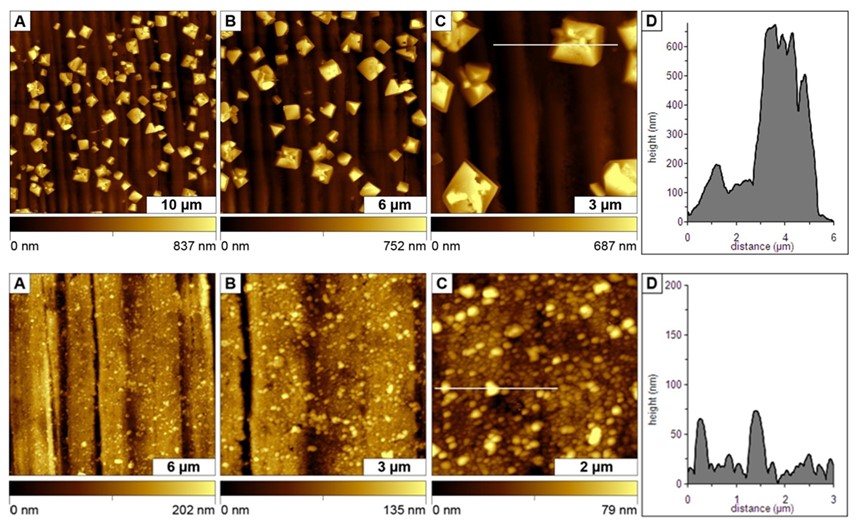
Surfaces changes after copper was exposed to water (top row) and after treatment with water containing orthophosphate (bottom row). Images were acquired after 6 h of treatment. [Daniels, S. L.; Kizilkaya, O.; Sprunger, P. T.; Lytle, D. A.; Garno, J. C. Nanoscale Surface Characterization of Aqueous Copper Corrosion: Effects of Immersion Interval and Orthophosphate Concentration. Applied Surface Science, 2013, 285P, 823– 831.]
Surface Assembly of Porphyrin-Cobaltacarborane Conjugates (Vicente, LSU)
The morphology and transitions of crystal assembly were captured in a single AFM image to investigate how porphyrin molecules aggregate on surfaces. The successive evolution of the stages of crystal growth can be visualized by organizing the changes in morphology with increasing size of aggregates. The smallest structures are the spherical seed particles, which begin to organize into linear and then columnar shapes. The linear columns then grow larger by assembling laterally into rectangular 2D sheets. As the sheets become larger, branching takes place from the centers of the planar sheets. Porphyrin crystals grow through assembly at the edges of the sheets in a planar direction. This study was done collaboratively with Professor Graca Vicente of LSU. [Hao, E.; Sibrian-Vazquez, M.; Serem, W.; Garno, J. C., Fronczek, F. R.; Vicente, M. G. H. Synthesis, aggregation and cellular investigations of porphyrin-cobaltacarborane conjugates. Chemistry, A European Journal, 2007, 13, 9035-9042.]
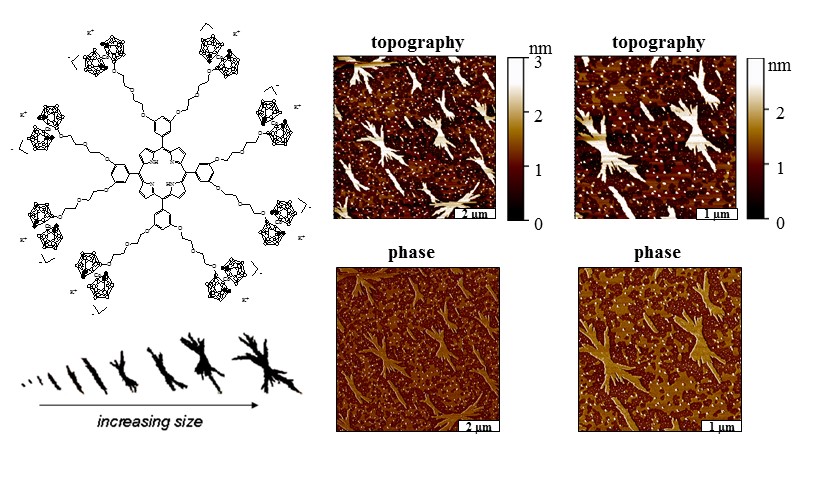 Conjugates of porphyrin-cobaltacarborane (top right) assemble on surfaces to form
crystallites. Tapping mode AFM reveals the fractal shapes of the crystals (top right)
with simultaneously acquired phase images (bottom right). The evolution of crystal
shapes is shown at the bottom, left providing insight about the successive growth
of crystal assemblies.
Conjugates of porphyrin-cobaltacarborane (top right) assemble on surfaces to form
crystallites. Tapping mode AFM reveals the fractal shapes of the crystals (top right)
with simultaneously acquired phase images (bottom right). The evolution of crystal
shapes is shown at the bottom, left providing insight about the successive growth
of crystal assemblies.
Studies of the Growth, Evolution and Self-aggregation of β-amyloid Fibrils (Hammer, LSU)
Amyloid peptide (Aβ) is the major protein component of plaques found in Alzheimer’s disease, and the aggregation of Aβ into oligomeric and fibrillic assemblies has been shown to be an early event of the disease pathway. In collaborative studies with Prof. R. P. Hammer of LSU, visualization of the progressive evolution of nanoscale changes in the morphology of Aβ oligomeric assemblies and amyloid fibrils was investigated using atomic force microscopy (AFM). The early stages of Aβ growth showed a predominance of spherical seed structures, oligomeric assemblies, and protofibrils; however the size and density of fibrils progressively increased with time. Within a few days of incubation, linear assemblies and fibrils became apparent. Over extended time scales of up to 5 months, the fibrils formed dense ensembles spanning lengths of several microns, which exhibit interesting changes due to self-organization of the fibrils into bundles or tangles. Detailed characterization of the Aβ assembly process at the nanoscale will help elucidate the role of Aβ in the pathology of Alzheimer’s disease.
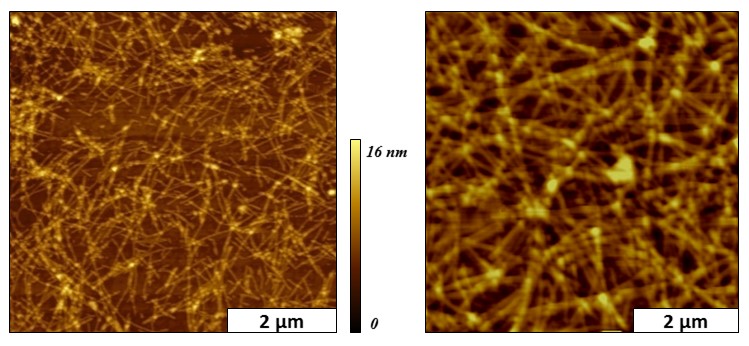 Snapshots of mature beta amyloid fibrils viewed with tapping mode AFM. [Serem, W.
K.; Bett, C. K.; Ngunjiri, J. N.; Garno, J. C. Studies of the growth, evolution and
self-aggregation of β-amyloid fibrils using tapping-mode atomic force microscopy.
(invited) special issue about NanoBio Imaging and Analysis. Microscopy Research and
Technique, 2010, 74(7), 699-708.
Snapshots of mature beta amyloid fibrils viewed with tapping mode AFM. [Serem, W.
K.; Bett, C. K.; Ngunjiri, J. N.; Garno, J. C. Studies of the growth, evolution and
self-aggregation of β-amyloid fibrils using tapping-mode atomic force microscopy.
(invited) special issue about NanoBio Imaging and Analysis. Microscopy Research and
Technique, 2010, 74(7), 699-708.
Polymeric gel containing fiber structures imaged with FMM (Zhang, LSU)
Features that cannot be resolved within topography frames can be clearly distinguished in phase and amplitude frames when using the AFM configuration of force modulation microscopy (FMM). A piezoceramic actuator is placed underneath the sample for FMM. [Lu, L.; Xu, S.; Zhang, D.; Garno, J. C. Sample stage designed for force modulation microscopy using a tip-mounted AFM scanner. Analyst 2016, 141, 1753 – 1760. ] The amplitude and phase of the AC waveform applied to the piezo will drive the entire sample to vibrate. Phase and amplitude mages map the differences in hardness and softness of materials. In collaboration with Prof. Donghui Zhang of LSU, samples of a polymeric gel containing embedded fibers were imaged using FMM.
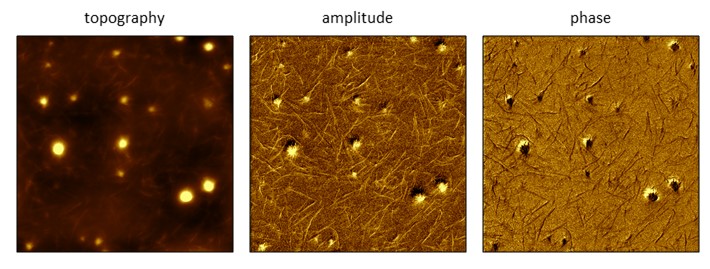
Polymeric gel sample containing fibrils imaged with FMM (8 × 8 μm2). The fibrils cannot be clearly resolved in the topography frame, however the amplitude and phase channels capture the fine details of fibril structures and arrangements. Images were acquired in ambient air, at a frequency of 154.2 kHz.