Research Interests
We are ecologists and evolutionary biologists who aim to better understand how the natural world works. Curiosity and unsolved real-world problems motivate us to characterize patterns in nature and to advance or evaluate scientific hypotheses for the processes (a.k.a. mechanisms) that cause, create, maintain, or modify those patterns. In these empirical-evidence-based pursuits, our attention is especially drawn toward the hierarchical levels of biological organization that range from organisms – through populations and communities – to ecosystems, including the global biosphere. We primarily study phenomena that intrigue us as naturalists and natural historians, or that concern us as conservation biologists, environmentalists, and engaged global citizens who strive for conservation of biodiversity, social and environmental justice, and planet-wide sustainability.
Groundcover Diversity & Dynamics in Longleaf Pine Ecosystems
of the Subtropical Southeastern United States of America
 Balduina uniflora (Asteraceae) — Abita Springs, Louisiana, USA
Balduina uniflora (Asteraceae) — Abita Springs, Louisiana, USA
 Prescribed fire — Camp Whispering Pines, Louisiana, USA; photo © C. E. T. Paine
Prescribed fire — Camp Whispering Pines, Louisiana, USA; photo © C. E. T. Paine
 Quadrat with Sarracenia alata (Sarraceniaceae) — Abita Springs, Louisiana, USA
Quadrat with Sarracenia alata (Sarraceniaceae) — Abita Springs, Louisiana, USA
Remnant and restored longleaf pine (Pinus palustris [Pinaceae]) ecosystems (savannas, flatwoods, sandhills, etc.) of the southeastern United States often support extreme levels of groundcover – mostly herbaceous – biodiversity. We have contributed toward the overall understanding of these extraordinary communities through observations and experiments on private and public lands in Florida (e.g., Eglin Air Force Base) and Louisiana (e.g., Camp Whispering Pines Girl Scout Camp and two of The Nature Conservancy's protected areas: Abita Creek Flatwoods Preserve & Lake Ramsay Preserve).
Our basic-science research on community assembly in pine savannas helps contextualize and quantify the effects of dispersal limitation, fire conditions, and species interactions on the high-diversity groundcover (e.g., Gagnon et al. 2012; Harms et al. 2017; Myers & Harms 2009, 2011). In addition, we found that the few native bunchgrass species that physically dominate the groundcover – as a guild of foundation species – tend to be over-dispersed (i.e., nearest-neighbor individuals tend to be farther away than expected by chance; Hovanes et al. 2018). This spatial-patterning natural-history phenomenon is a key to understanding these high-diversity communities. Specifically, since the dominant bunchgrasses do not form continuous lawns, diverse smaller-stature forbs and grasses establish and persist in the interstices among the dominant-grass tussocks, yet with limited opportunities to outcompete one another.
Our fire-ecology research implicates soil heating as a principal driver of groundcover assembly (Gagnon et al. 2015). In addition, extreme flammability of above-ground organs in many plants from fire-prone ecosystems may provide adaptive protection to underground regenerative tissue from lethal heating during fires (Gagnon et al. 2010). We proposed through our pyrogenicity-as-protection adaptive hypothesis (a.k.a. save-thyself hypothesis) that highly flammable plants burn quickly, which reduces the duration of soil heating, by which plants protect their underground perennating organs or offspring as seeds in the soil seedbank. Our idea is a mutually compatible alternative to the kill-thy-neighbor hypothesis (Bond & Midgley, 1995, Oikos). In other words – one, or the other, or both – could favor the evolution of highly flammable plants.
A concept that emerged from our work in longleaf ecosystems and which we think has broad global applicability is the idea that Species of Unusual Effect (SUEs) deserve special attention to understand community assembly and ecosystem functions (e.g., productivity, nutrient cycling, etc.), as well as for conservation and restoration (Harms 2022). SUEs comprise keystone species, foundation species, ecosystem engineers (at least those with extraordinary influence), and any other species that play outsized roles in either community assembly or ecosystem functions. For example, at Camp Whispering Pines, we found that discrete groundcover patches dominated by bracken fern (Pteridium aquilinum) or gallberry (Ilex glabra) have significantly reduced species diversity relative to most of the groundcover that is dominated by foundational native bunchgrasses (Barker & Harms 2025). Accordingly, our working hypothesis is that as alternative local foundation species and relative to one another at Camp Whispering Pines, native bunchgrasses are diversity-enhancing SUEs, whereas bracken fern and gallberry are diversity-inhibiting SUEs – all of which add pattern-diversity to the overall landscape.
To facilitate global geographic comparisons among grasslands (ecosystems whose groundcover is dominated by grasses or other graminoids), Bret Elderd, Jennie Kluse, and I shared our Lake Ramsay observational dataset – cover values per species for thirty 1-m2 quadrats – with the globally distributed Nutrient Network (our NutNet site name = "TNC Lake Ramsay Preserve"; our NutNet site code = "rams.us"). For example, from a global comparison of NutNet sites, we found that intense solar radiation appears to act as a strong ecological filter in grassland community assembly (Spohn et al. 2026).
We also capitalize on opportunities to apply and translate our science toward conservation and restoration in longleaf pine ecosystems, especially through educational and management-based outreach. For example, see my essays in The Longleaf Alliance's quarterly magazine, The Longleaf Leader (Harms 2019, re the ecological importance of native bunchgrasses; Harms 2023, re the old-growth concept applied to southeastern U.S. grasslands). Also see our workshop report (Harms et al. 2015) with recommendations to the Partnership Council of America's Longleaf Restoration Initiative.
We thank Eglin Air Force Base (especially Jackson Guard), the Girl Scouts of America (specifially the Girl Scouts Louisiana East), the National Science Foundation (grants DEB #0516175, DEB #114408), and The Nature Conservancy for supporting our projects.
Rainforest Diversity & Dynamics in Queensland, Australia
 Joseph H. Connell — Far North Queensland, Australia; photo © P. T. Green
Joseph H. Connell — Far North Queensland, Australia; photo © P. T. Green
 2013 (50th anniversary) census crew under the "Lunch Tree" on Connell's Davies Creek plot; photo
© P. T. Green
2013 (50th anniversary) census crew under the "Lunch Tree" on Connell's Davies Creek plot; photo
© P. T. Green
 2023 (60th anniversary) census crew under the "Lunch Tree" on Connell's Davies Creek plot; photo
© P. T. Green
2023 (60th anniversary) census crew under the "Lunch Tree" on Connell's Davies Creek plot; photo
© P. T. Green
During a more than half-century-long, highly productive career, our inspirational mentor and friend, Joe Connell (1923-2020; see Sousa et al. 2021), established long-term research projects in Australian rainforests (e.g., Connell et al. 2005) and on the Great Barrier Reef (e.g., Connell et al. 2004), among others. Joe initiated his forest dynamics project by establising permanent plots in tropical (Davies Creek & Dinden National Parks) and subtropical (Lamington National Park) Queensland, Australia in 1963. Pete Green (La Trobe University, Australia) inherited and currently oversees the Connell-legacy rainforest plots project, a cornerstone of the Queensland Permanent Rainforest Plots network. In 2013, we conducted the 50th anniversary census of the trees in Connell's plots (Harms & Green 2014); in 2023, we conducted the 60th anniversary census (see my third place winning entry entitled "Rosa's Tree Hug" – taken during the census – on the website for the 2024 joint photo contest of the Association for Tropical Biology & Conservation and Society for Tropical Ecology).
Using the Davies Creek (Queensland Wet Tropics) demographic data, we found empirical support for the long-standing idea in forest ecology that early-stage (e.g., seed and seedling) dynamics are especially consequential to forest composition and diversity (Green, Harms & Connell 2014). These observations motivated us to develop conceptual models and mechanistic hypotheses concerning disproportionate non-random mortality among life-cycle stages in all types of communities (Green & Harms 2018).
In a first step toward better understanding the microbial influence on recruitment at Davies Creek, Jen Wood selectively sampled microbial assemblages in Connell's plot, and found microbial compositional patterns associated with non-random, diversifying seedling dynamics (Wood et al. 2020).
Global Patterns of Neighborhood-Dependent Demographic Performance: Influences of Neighbor Density, Distance & Phylogenetic Relatedness on Individual Trees & Lianas
 Healthy, unattacked Protium stevensonii (Burseraceae) seedling — BCI, Panama
Healthy, unattacked Protium stevensonii (Burseraceae) seedling — BCI, Panama
 Attacked, pit-galled Protium stevensonii (Burseraceae) seedling — BCI, Panama
Attacked, pit-galled Protium stevensonii (Burseraceae) seedling — BCI, Panama
 Pit-galling hemipterans on Protium stevensonii (Burseraceae) — BCI, Panama
Pit-galling hemipterans on Protium stevensonii (Burseraceae) — BCI, Panama
Stemming from their stay-at-home lifestyles, individual plants tend to interact primarily with their near neighbors. Accordingly, the demographic consequences of neighborhood interactions have the potential to scale up to more broadly shape community assembly (e.g., maintain diversity, organize patterns of alpha- and beta-diversity, etc.). Our analyses of spatially detailed demographic records – especially for woody species in mapped forests – clearly indicate that non-random, neighborhood-scale, inter-individual interactions influence individual-level performance (growth, mortality, recruitment) – often in ways that could foster coexistence, as well as contribute toward the origins and maintenance of biodiversity.
Among other patterns, we have found:
(1) negative conspecific density-dependent seed-to-seedling transitions among common species of trees and lianas in the Center for Tropical Forest Science (CTFS – a research branch of the Smithsonian Tropical Research Institute [STRI], now ForestGEO) 50-ha Hubbell-Foster Forest Dynamics Plot (FDP) on Barro Colorado Island (BCI), Republic of Panama, which is consistent with the Janzen-Connell hypothesis for the maintenance of diversity in high-diversity plant communities (Harms et al. 2000);
(2) density- and distance-dependent survival of seedlings and small saplings of a common canopy tree species on BCI (Gilbert et al. 2001), which is also consistent with the Janzen-Connell hypothesis;
(3) between-seedling distances too large for competition among conspecific seedlings to play a substantial role in early-stage, non-random seedling mortality at Cocha Cashu Biological Station, Peru (Paine et al. 2008);
(4) reduced survival among established trees in conspecific-rich neighborhoods (with relatively consistent patterns among 5 CTFS FDPs in Asia and 2 in the Neotropics; Wills et al. 2006);
(5) and neighborhood consequences for individual-level performance of saplings and established trees that generally – albeit idiosyncratically – taper off with phylogenetic distance separating the focal stem from its neighbors (Wills et al. 2016; Wills et al. 2021), based on our novel Equal-Area Annulus (EAA) point-pattern statistical method, which employs a stems'-traits-swapping randomization or null model, and which we have applied to several CTFS FDPs. (Sub-heading #6 in the Discussion of Wills et al. (2016) was not supposed to say "resource use." This unfortunate publication error obscures our aim to contrast the evolutionary phenotypic convergence - especially of resource-acquisition traits - expected when resources are shared among diverse tree taxa in unpredictable neighborhoods vs. the expected evolutionary divergence among tree taxa that share enemies, such as fungal pathogens or herbivores. Divergence is expected in traits whose expression shifts the enemy's attention or effectiveness away from the focal taxon or toward heterospecific neighbors; in many cases these could be defense traits. In other words, shared enemies should favor phenotypic divergence at any given degree of phylogenetic distance between two taxa.)
Several other research groups are improving our understanding of these neighborhood spatiotemporal phenomena with detailed work on the mechanisms (e.g., fungal pathogen infection, herbivory, vertebrate seed predation) that cause the patterns. (See also the previous section on this webpage regarding Jen Wood's work on microbial assemblages associated with the early life-cycle stage distance- and density-dependence that we had previously quantified in Connell's Davies Creek forest dynamics plot in Queensland, Australia.) My own observations in Panama suggest that a key culprit causing non-random (possibly both density- and distance-dependent) early-stage mortality in Protium stevensonii (which went by Tetragastris panamensis when I first met this species; Burseraceae) – one of the most common canopy trees on the BCI FDP – is a somewhat host-specific pit-galling hemipteran that appears to "rain" down from conspecific adult canopies; this is one of the key mechanisms Janzen imagined when he proposed his version of the Janzen-Connell hypothesis.
Sodium in Phytocationic Landscapes and
as a Keystone Element for Community Ecology

Lepidopterans puddling (probably for sodium) among riverbank peccary bones at Cocha Cashu, Peru

Luis's hydroponic setup to precisely manipulate sodium in Helianthus annuus (Asteraceae)

Luis's bordered patch butterfly (Chlosyne lacinia [Nymphalidae]) rearing containers
Adriana Bravo's experiments and observations at the Los Amigos Biological Station in lowland Peru supported our hypothesis that frugivorous stenodermatine bats (a subfamily of leaf-nosed bats, Phyllostomidae) visit collpas (clay licks) to obtain sodium that is otherwise in short supply in their diets (Bravo et al. 2008, 2010a, 2010b, 2012).
Adriana's bat project expanded into an investigation of the biogeography of sodium availability in keystone resources (fig [Ficus] fruits) throughout the Neotropics. For the project, Adriana obtained dried fruit specimens from herbaria, curated from collections across Central and South America (Bravo & Harms 2017). Our observations concerning orders-of-magnitude differences among sites in sodium availability to frugivores, as well as the widespread geographic patterning of extremely low-sodium fruits, cause me to think of sodium as a "keystone element" in community ecology: it is disproportionately important to consumers – far beyond its often meager availability to them.
Luis Santiago-Rosario's systematic literature review and analysis of 107 plant populations (67 species in 20 plant families) grown under controlled laboratory and greenhouse conditions, demonstrated that even though plants vary in their growth responses to substrate-sodium concentrations, plant tissues generally become monotonically saltier as substrate saltiness increases (Santiago-Rosario et al. 2021). This observation gave rise to our no-escape-from-sodium statistical hypothesis, by which we predicted that on average plants in saltier soils in nature have saltier tissues. To test the prediction and to better understand variation in plant-tissue sodium for organisms at higher trophic levels, Luis's project expanded into an investigation of phytocationic landscapes (e.g., Santiago-Rosario et al. 2022; this is Mark Hunter's phytochemical landscape concept applied to cations).
In addition to his aforementioned work on the sodium-relationships between plants and their substrates, Luis also studied several aspects of the sodium-relationships between plants and their herbivores. For example, he designed hydroponic methods to relatively precisely produce sunflower host plants of varying levels of saltiness to ask how the bordered patch butterfly (Chlosyne lacinia [Nymphalidae]) responded behaviorally and physiologically. (For an overview of the natural history of this extraordinary species, see Phelps et al. 2023). Later, he compared and contrasted the physiological results with simliar experiments on three additional butterfly species (Santiago-Rosario et al. 2025). One dramatic behavioral consequence of C. lacinia's hydroponically-manipulated low-sodium diets was sibling cannibalism among these otherwise herbivorous caterpillars (Santiago-Rosario et al. 2023).
Maggie Vincent also investigated the influence of larval sodium availability to C. lacinia, specifically adult mating behavior, reproduction, and mortality risk (Vincent et al. 2025). To control larval access to sodium, she used a hydroponic set-up similar to Luis's, then observed the adult butterflies' courtship and mating behavior, measured females' fecundity, and determined lengths of adult lifespans. Mortality was clearly elevated for butterflies reared on the highest larval host-plant concentrations of sodium, and some aspects of adult mating behavior varied as a function of their larval access to sodium.
Soil-Borne Plant Resources Shape Diversity, Dynamics & Structure of Forests Worldwide
 Soil chemical extractions — INPA Soils Lab, Manaus, Brazil
Soil chemical extractions — INPA Soils Lab, Manaus, Brazil
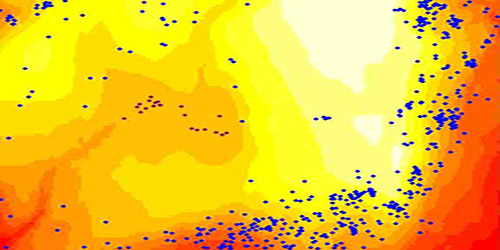 BCI, Panama 50-ha Hubbell-Foster CTFS plot topography (longer wavelength colors =
lower elevations); together with two species' spatial distributions (blue dots = Ocotea whitei [Lauraceae; positively associated with the eastern and southern slopes]; red dots
= Elaeis oleifera [Arecaceae; positively associated with the swamp])
BCI, Panama 50-ha Hubbell-Foster CTFS plot topography (longer wavelength colors =
lower elevations); together with two species' spatial distributions (blue dots = Ocotea whitei [Lauraceae; positively associated with the eastern and southern slopes]; red dots
= Elaeis oleifera [Arecaceae; positively associated with the swamp])
Plant distribution patterns are often biased with respect to environmental variables (e.g., soil chemistry, moisture) or landscape features (e.g., topography). The nature of the biases, especially the extent to which they differ among species and their underlying causes, helps sort among alternative mechanisms for the maintenance of species diversity and other emergent properties of community assembly.
Collaborators and I have found distributional biases in several of the Center for Tropical Forect Science (CTFS – a research branch of the Smithsonian Tropical Research Institute [STRI]; now ForestGEO) Forest Dynamics Plots (FDPs). To assess patterns of association between mapped stems and delineated habitats, I developed the novel Torus-Translation Test – a point-pattern statistical method that employs a toroidal randomization or null model (Harms 1997). We published Torus-Translation Test results (e.g., that many species share simliar habitat-association patterns) using data from the Barro Colorado Island (BCI), Republic of Panama FDP (Harms et al. 2001). Collaborators and I also found shared distributional biases with respect to topography in the Korup, Cameroon FDP (Chuyong et al. 2011) and the Sinharaja, Sri Lanka FDP (Gunatilleke et al. 2006). In similar studies, we found biased distributions with respect to soil types among lianas in Sabah, Malaysia (DeWalt et al. 2006) and with respect to soil and topographic variables among trees in the Brazilian Amazon (Laurance et al. 2010).
To better understand the underlying processes that generate spatial patterns of tropical tree and liana distribution, diversity, and dynamics, Jim Dalling (University of Illinois), Robert John-Chandran (Indian Institute of Science Education & Research), Joe Yavitt (Cornell University), Bob Stallard (U. S. Geological Survey), and I systematically characterized soil properties and hydrology of selected CTFS FDPs. Our soil project stimulated similar assessments by other research groups in many of the temperate and tropical CTFS FDPs. Soil-chemical and hydrologic spatial patterns help explain many individual species' distributional patterns and assemblage-level compositional patterns in forests throughout the world (e.g., Baldeck et al. 2013a, b, c, 2016 [trees at BCI and 7 other African, Asian & Neotropical FDPs]; Dalling et al. 2012 [lianas at BCI]; John et al. 2007 [trees at BCI and 2 other Neotropical FDPs]).
In the BCI centennial volumes, I showed that "chance alone cannot account for patterns of habitat association on the BCI FDP" (Harms 2024, pg. 309). By extension, if patterns of habitat association reflect underlying specialization (which is, admittedly, only one possible mechanism for the patterns), chance alone could not account for patterns of relative abundance, since taxa restricted to rare habitat types could not be relatively more common in their communities than their preferred habitats are in those communities.
We thank the National Science Foundation (grants DEB #0211004, OISE #0314581) and the Smithsonian Tropical Research Institute for supporting our initial soil and hydrology collaborative effort, including our soil-sampling, soil-analysis, and data-analysis training workshops in Panama, which expanded sampling and assessments to additional sites within the CTFS network and beyond.
Nutrient Cycling & Limitation in a Lowland Old-Growth
Tropical Forest on the Isthmus of Panama
 Gigante Peninsula Forest Fertilization Experiment, Panama
Gigante Peninsula Forest Fertilization Experiment, Panama
 Rain-shelter denizen
Rain-shelter denizen
 "Green" & "brown" food webs — Gigante Peninsula, Panama
"Green" & "brown" food webs — Gigante Peninsula, Panama
To test a variety of predictions concerning nutrient cycling and limitation of lowland tropical forests, since 1998 we have been adding nutrients to 40x40-m plots of relatively old-growth forest on the Gigante Peninsula of the Barro Colorado Nature Monument of the Smithsonian Tropical Research Institute (STRI), Republic of Panama (Santiago et al. 2012; Sayer et al. 2012; Woods et al. 2018; Wright et al. 2011; Yavitt et al. 2009, 2011). We have four replicates of an NPK-factorial design, plus separate micronutrient plots.
Key results from the experiment support the growing awareness of multi-nutrient limitation of ecosystem processes (e.g., decomposition, Kaspari et al. 2008; components of productivity, Wright et al. 2024) and the limited responsiveness of old-growth forests to anthropogenic nutrient additions (at least over several years to a few decades, i.e., short time scales relative to tree lifetimes; Wright et al. 2018).
Joe Wright (STRI), Joe Yavitt (Cornell University), and I conceived the idea and designed the project; then together with Milton García we initiated fieldwork in 1997 (to determine baseline conditions prior to nutrient amendments). As Joe Wright has maintained the project from his base at STRI, several ecologists, ecophysiologists, and biogeochemists have used our experiment as a springboard from which to further advance our understanding of nutrient cycling and limitation in tropical forests, including: Mike Kaspari (University of Oklahoma), Jennifer Powers (University of Minnesota), and Lou Santiago (University of California at Riverside), among others.
We thank the Smithsonian Institution and the Smithsonian Tropical Research Institute for supporting this project.
Diversity & Dynamics in Intact & Fragmented Tropical Forests
of Central Amazonian Brazil
 Ale Oliveira identifying a leaf in our central Amazonian CTFS plot
Ale Oliveira identifying a leaf in our central Amazonian CTFS plot
 Undescribed ("new to science") species of shrub (Violaceae) discovered in our central
Amazonian CTFS plot
Undescribed ("new to science") species of shrub (Violaceae) discovered in our central
Amazonian CTFS plot
 First census of our central Amazonian CTFS plot
First census of our central Amazonian CTFS plot
As the project's original Principal Investigators (PIs), Alexandre "Ale" Adalardo de Oliveira (Universidade de São Paulo, Brazil) and I spear-headed an effort to better understand forest diversity and dynamics in intact forests of the central Amazon Basin by adding a new node to the network of Forest Dynamics Plots (FDPs) coordinated by the Center for Tropical Forest Science (CTFS – a research branch of the Smithsonian Tropical Research Institute [STRI]; now ForestGEO). With abundant help from Liz Losos (who was then the Director of CTFS), Rita Mesquita (who was then the Scientific Director of the Biological Dynamics of Forest Fragments Project), and many others, we created a square, 49-ha (700x700-m) FDP within the continuous, old-growth forest north of Manaus, Brazil. Within the central, innermost 25-ha (500x500-m) square, all free-standing woody stems ≥1-cm diameter have since been mapped, measured, identified to species , and uniquely tagged for future re-measurements. Our FDP contains >1200 species of free-standing trees – more than any other ~50-ha forest diversity and dynamics research plot on Earth.
Ale and Alberto "Beto" Vicentini (Instituto Nacional de Pesquisas da Amazônia) now manage the project as its current PIs. Stand data from our Manaus FDP are being used in a variety of global comparisons among forests (e.g., Réjou-Méchain et al. 2014). Visit the Smithsonian Institution's Forest Global Earth Observatories (ForestGEO) web page of our Manaus FDP for a few additional details about the project. Also, keep abreast of ForestGEO developments through the ForestGEO blog and via the ForestGEO Flickr photostream. We thank the Brazilian Government, CTFS, ForestGEO, Louisiana Board of Regents, and Richard & Rhoda Goldman Fund for supporting our project.
In a separate, but coordinated endeavor, I collaborated with Bill & Sue Laurance (James Cook University, Cairns, Australia) and the Biological Dynamics of Forest Fragments Project (BDFFP) in Brazil to better understand changes that occur in Amazonian forests when they are anthropogenically fragmented (e.g., Laurance et al. 2006). With funding from the National Science Foundation (grant DEB #0614044), we conducted the first comprehensive census of sapling recruits (1 to 10-cm diameter) in subplots within the 1-ha phytodemographics plots, which are strategically located in fragments and associated continuous forests across the BDFFP landscape.
Adaptive & Sexually-Selected Evolution of Plant Traits,
Especially Owing to Their Interactions with Other Species
(Herbivores, Pollinators, Seed Dispersers, etc.)
 Seed of Mora excelsa (Fabaceae) — Suriname
Seed of Mora excelsa (Fabaceae) — Suriname

Flower of Hibiscus aculeatus (Malvaceae) — Louisiana, USA

Flowers of Amyema quandang (Loranthaceae) — Australia
Unusual organismal traits beg for ultimate explanations, i.e., why (in an evolutionary sense) do they exist? Such explanations require understanding the selective consequences of the traits for the organisms that possess them. In the first section on this webpage, I gave the example of plants with highly flammable tissues that thrive in fire-frequented ecosystems. In subsequent sections, I give two further examples: "trash basket" architecture for nutrient acquisition in a tropical montane treelet and water calyces for floral defense in a tropical lowland herbaceous plant.
My earliest contributions – with Jim Dalling (University of Illinois) – toward better understanding adaptive traits in plants concerned the importance of resprouting to the recruitment ecology and life-history evolution of large-seeded trees and lianas (e.g., Dalling et al. 1997; Dalling & Harms 1999; Harms et al. 1997; Harms & Dalling 1997, 2024).
In the course of working with big seeds, I discovered a new species of clearwing moth (Carmenta foraseminis [Sesiidae]), whose larvae tunnel through the large seeds of Gustavia superba (Lecythidaceae) in central Panama (Harms & Aiello 1995). C. foraseminis has more recently emerged as a pest of cacao (Theobroma cacao, Malvaceae) in South America.
The large, exceptionally hard seeds of tagua or Panama ivory palm (Phytelephas seemannii) are used artisanally, especially by Wounaan craftspersons in Panama. We reviewed the natural history and uses of tagua for an article in the palm journal, Principes (Dalling et al. 1996).
Metha Klock examined nodulation and rhizobial symbioses in species of Acacia anthropogenically introduced to California from their native Australia, to test hypotheses about their differential influences on native Californian flora and ecosystems. Acacias appear to have been co-introduced to California along with their Australian rhizobial symbionts (Klock et al. 2022), but the evidence was mixed for differences in rhizobial promiscuousness by Acacia hosts on the extent of the plants' negative effects in their introduced ranges (Klock et al. 2015, 2016).
Many adaptive or sexually-selected plant traits influence the animals with which they interact. Seed dispersal, herbivory, pollination, etc. are all shaped by evolution, sometimes co-evolution (reciprocal evolution of each partner in response to the other), as well as learning and cultural transmission among the animal interactors. In this context, I am interested in the extent to which contrasting pollen on petals in some plants might affect the behavior of potential pollinators (Harms 2020).
Australia's showy mistletoes (Loranthaceae) express a variety of interesting traits. For example, some of the various hemiparasitic species develop flashy floral displays, yet are otherwise rather cryptic, i.e., they resemble their hosts (e.g., their leaves are shaped like their hosts' leaves; Harms et al. 2023). In addition, most have higher-quality, faster-turnover leaves than their hosts; accordingly, at least some appear to ramp-up local nutrient cycling in the immediate vicinities of their hosts. Sarah Mathews (LSU) initiated our collaboration to better understand the influences of species interactions on the evolution and expression of mistletoes' intriguing traits; collaborators include: Luis Santiago-Rosario (University of Minnesota), Arthur Porto (University of Florida), and Dave Watson (Charles Sturt University, Australia).
Collaborators and I have also explored several relationships among the functional, morphological, and physiological traits of plants, as well as their demographic characteristics (e.g., Poorter et al. 2008; I. J. Wright et al. 2007; S. J. Wright et al. 2010).
"Trash Basket" Trade-off: Light- vs. Nutrient-Capture
in Morisonia antonensis (Capparaceae)

Morisonia antonensis (Capparaceae) "trash basket" — Cerro Campana, Panama
 Altos de Campana National Park, Panama
Altos de Campana National Park, Panama

Morisonia antonensis (Capparaceae) — Cerro Campana, Panama
Morisonia antonensis (formerly Capparis antonensis , then Quadrella antonensis [Capparaceae]) is an unusual montane cloud forest treelet, endemic to the Republic of Panama. At our study site in Altos de Campana National Park, individuals sacrifice light-capture efficiency to obtain nutrients through aboveground adventitious roots that access decomposing leaf litter held in the "trash baskets" formed from the plant's own closely-spaced leaves (Harms, Dalling & Sánchez de Stapf 2020 – a Flora Obscura contribution to The New Phytologist Trust's Plants, People, Planet; enjoy the brief YouTube video summary). In our article, we compare and contrast litter trapping with botanical carnivory and certain types of myrmecophily as alternative nutrient-acquisition strategies. Jim Dalling (University of Illinois) and I worked on the basic litter-trapping biology. María Sánchez de Stapf (Universidad de Panamá) collaborated with us to complete a master's thesis by conducting a litter-removal experiment (which empirically demonstrated the performance advantage to these plants of trapping litter). We thank the Smithsonian Tropical Research Institute for supporting this project.
Water Calyces Dissuade Specialist Floral Enemies
in Chrysothemis friedrichsthaliana (Gesneriaceae)
 Chrysothemis friedrichsthaliana (Gesneriaceae) — BCI, Panama; photo © G. Dimijian
Chrysothemis friedrichsthaliana (Gesneriaceae) — BCI, Panama; photo © G. Dimijian
 Alucita sp. cf. flavicincta (Alucitidae) — Osa Peninsula, Costa Rica; photo © J. E. Carlson
Alucita sp. cf. flavicincta (Alucitidae) — Osa Peninsula, Costa Rica; photo © J. E. Carlson
Chrysothemis friedrichsthaliana (Gesneriaceae) develops water calyces, i.e., upright calyces that fill with water which covers the additional floral parts during their early development. Jane Carlson's experiments on the Osa Peninsula of Costa Rica demonstrated that flowers with experimentally drained calyces suffered higher rates of attack by a microlepidopteran floral herbivore (Alucita sp. cf. flavicincta [Alucitidae]), supporting the adaptive hypothesis that water calyces dissuade specialist floral enemies (Carlson & Harms 2007). Jane also discovered that this plant biases nectar production toward the male phase of its protandrous flowers; she subsequently reviewed the phenomenon of gender-biased nectar production in dichogamous flowering plants (Carlson & Harms 2006).
Behavioral Ecology & Evolution
 Guanacos (Lama guanicoe) — Torres del Paine, Chile
Guanacos (Lama guanicoe) — Torres del Paine, Chile
 Yellow-headed blackbird (Xanthocephalus xanthocephalus) — Columbia National Wildlife Refuge, Washington, USA
Yellow-headed blackbird (Xanthocephalus xanthocephalus) — Columbia National Wildlife Refuge, Washington, USA
 Jessica Eberhard & monk parakeet (Myiopsitta monachus) — Entre Ríos, Argentina
Jessica Eberhard & monk parakeet (Myiopsitta monachus) — Entre Ríos, Argentina
Even though most of my current research has a plant-ecology focus, I have an abiding interest in behavioral ecology. Natural history has aroused my curiosity and sense of wonder for as long as I can remember. Nearly any aspect of an organism's natural history can grab and hold my attention; even so, as a university undergraduate student, I was especially intrigued by the behavior of animals in the wild. For example, during a 7-month, personally transformative experience in Torres del Paine National Park, Chile, I was a field assistant to Bill Franklin and Warren Johnson (who were then professor and Ph.D. student, respectively, at Iowa State University). I helped study the foraging and social behavior, as well as interspecific interactions, of some of the park's carnivores (two species of fox [Lycalopex culpaeus; L. griseus]; Patagonian hog-nosed skunk [Conepatus humboldtii]; Geoffroy's cat [Leopardus geoffroyi]; and puma [Puma concolor; see Bill's 1991 National Geographic article – I am the "student assistant" on pg. 109]). I also completed an undergraduate honors thesis from my investigation of sex-biased yearling dispersal of guanacos (Lama guanicoe) during the 1988 birthing season in the long-term study population. One of Bill's previous research teams had ear-tagged many of the yearling guanacos the year before, which facilitated our observations of their movements during the season of my study. Generally, yearling males dispersed considerably farther than yearling females when a given yearling was chased away from its mother's family group by the group's adult territorial male.
Just prior to graduate school, I continued to reinforce my passion for field biology as a field assistant to Gordon Orians and Les Beletsky (who were then professor and post-doc, respectively, at the University of Washington). I contributed toward their long-term investigation of the courtship, mating, nesting, and parental behavior of three species of icterid blackbirds (red-winged [Agelaius phoeniceus]; Brewer's [Euphagus cyanocephalus]; and yellow-headed [Xanthocephalus xanthocephalus]) in the pothole cattail marhes of eastern Washington (Harms et al. 1991).
In graduate school and beyond, I have enjoyed tagging along on some of Jessica Eberhard's research trips. During one of those trips, we documented the communal roosting behavior of indigenous parrots, doves, and pigeons on Aruba, Bonaire, Curaçao, and Isla Margarita, Venezuela (Harms & Eberhard 2003).
Sandra Galeano's experiments with and observations of the highly polymorphic / polytypic frog, Oophaga pumilio, in the Bocas del Toro Archipelago, Republic of Panama supported our prediction that males from aposematic, brightly-colored populations would be more aggressive toward both conspecific and heterospecific males than would individuals from cryptic populations (Galeano & Harms 2016). Sandra's project helps link the evolution of behavioral and morphological traits in O. pumilio via natural and sexual selection to their community-level contexts and consequences.
Right in my own backyard, for at least six out of seven consecutive years, I observed male two-spotted longhorn bees (Melissodes bimaculatus) roost in clusters in the same small iris patch. On a few occasions, parasitic cuckoo bees (Triepeolus lunatus) co-roosted with the longhorn bees, which prompted me to wonder whether any of the bees might glean useful information from roostmates to inform their next day's activities (an extension of Ward & Zahavi's Information Center Hypothesis) (Harms & Owens 2025).
Thank you!
I am grateful to my many collaborators (students, post-docs, professors, researchers, field assistants, and other friends and members of my family) who have helped me nurture a wide range of interests during my thoroughly enjoyable research career!