Research
Background
Neurohypophysial Hormones, Vasopressin and Oxytocin
Both vasopressin and oxytocin are nonapeptides (9 amino-acids long) and released from the posterior pituitary (neurohypophysis) into the general circulation in response to physiological demands. Although molecular structure of these hormones only differs by two amino acid sequences out of nine at third and eighth locations, they induce very different physiological activities. Vasopressin, also known as arginine vasopressin (AVP) or anti-diuretic hormone (ADH), produces antidiuretic and vasoconstriction effects during osmotic and cardiovascular challenges. Oxytocin plays important roles in stimulating milk release and parturition.
Sequences of Vasopressin and OxytocinVasopressin: Cys-Tyr-Phe-Gln-Asn-Cys-Pro-Arg-Gly- NH2Oxytocin: Cys-Tyr-Ile-Gln-Asn-Cys-Pro-Leu-Gly-NH2
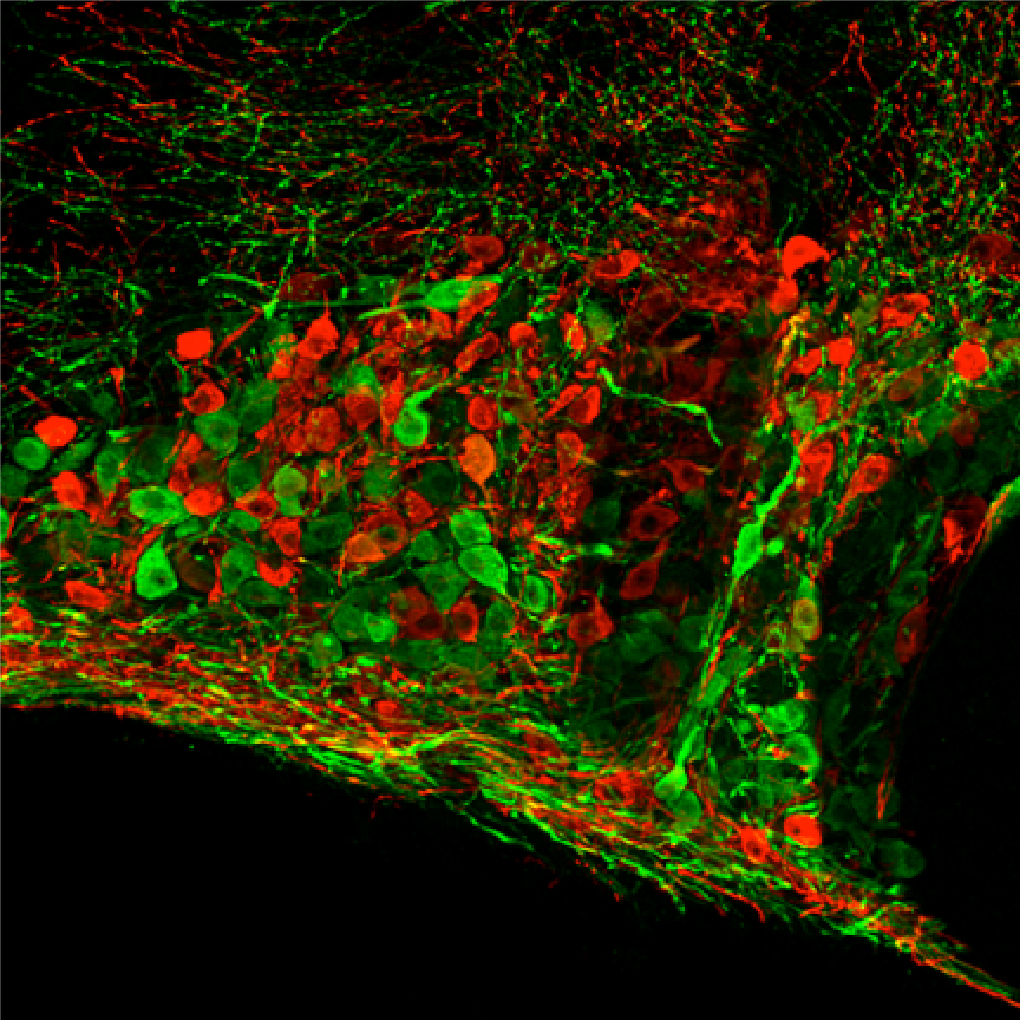 Fig. 1. Immunocytochemically localized Oxytocin (Red) and Vasopressin (Green) neurons in
the supraoptic nucleus of the hypothalamus
Fig. 1. Immunocytochemically localized Oxytocin (Red) and Vasopressin (Green) neurons in
the supraoptic nucleus of the hypothalamusSynthesis of Vasopressin and Oxytocin
Vasopressin and oxytocin are synthesized by magnocellular neurosecretory neurons (We call them "Magno cells" in our lab) located in the supraoptic nucleus (SON) and paraventricular nucleus (PVN) in the hypothalamus. Intriguingly, most Magno-cells predominantly synthesize one of these hormones, either vasopressin or oxytocin, although vasopressin- and oxytocin-neurons appear morphologically identical (Fig. 1).
Release of Vasopressin
The release of oxytocin and vasopressin hormones from the neurohypophysis depends largely on the frequency and pattern of action potentials of their synthesizing neurons. Vasopressin neurons respond to hyperosmolality, hypovolemia, and hypotension by increasing their firing rate and adopting a "phasic bursting" pattern comprising alternating periods of activity (7-15 Hz) and silence, each lasting tens of seconds (Fig. 2).
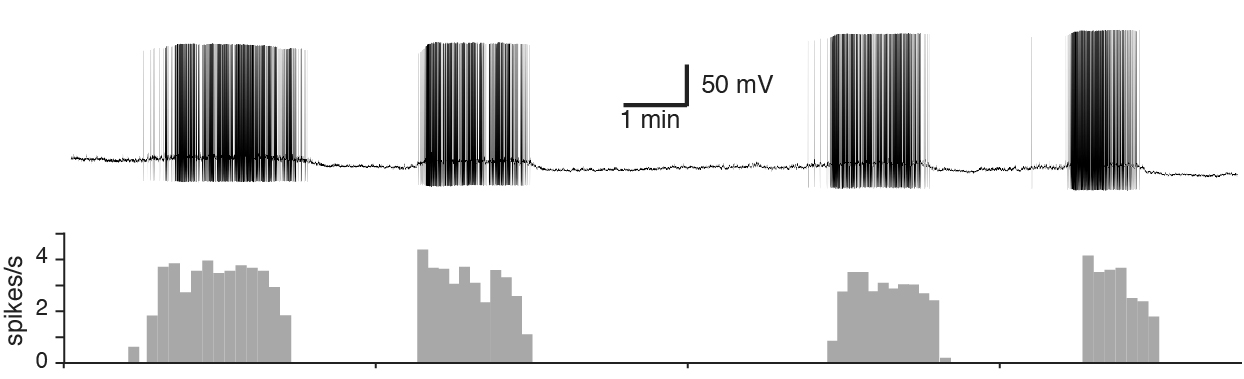 Fig. 2. Whole-cell Patch-Clamp recording of the phasic bursting activity of a vasopressin
neuron. The phasic bursting activity of vasopressin neurons are observed in in vitro
brain slice preparations making vasopressin neurons amenable subjects for studying
the functional significance of their electrophysiological properties in hormone secretion.
Fig. 2. Whole-cell Patch-Clamp recording of the phasic bursting activity of a vasopressin
neuron. The phasic bursting activity of vasopressin neurons are observed in in vitro
brain slice preparations making vasopressin neurons amenable subjects for studying
the functional significance of their electrophysiological properties in hormone secretion.
Release of Oxytocin
Preceding each milk ejection, the expression of the firing pattern in oxytocin neurons changes dramatically from basal spontaneous firing to synchronized short bursting activity characterized as short (2-4 seconds) and high frequency (up to 80 Hz). This synchronized short-bursting activity of oxytocin neurons results in a bolus release of oxytocin. Perhaps, this is important for inductions of cyclic, quick contractions of the mammary gland during milk ejection and uterus during parturition.
The changes in firing frequency and pattern of action potentials in the synthesizing neurons are not only important for the quantity of the neurohypophysial hormones released, but also for the appropriate physiological effects of these hormones. Nevertheless, the occurrence of the firing pattern is an essential part of the response of these neurons to the physiological demand.
Factors Regulating the Electrical Activity
The electrical activity of a neuron is regulated by the synaptic properties and intrinsic membrane properties (i.e. ion channels/pumps) of the neuron. Thus, the physiological demands influence these properties to control the electrical activity of Magno-cells, so that optimum amounts of hormones for the particular moment are released into the general circulation. Alternatively, abnormal expression of these properties leads to abnormal firing frequency and/or pattern of action potentials, which in turn results in irregular secretions of these hormones.
Vasopressin is the main antidiuretic hormone and a major vasoconstrictor in mammals, and a basic understanding of the regulation vasopressin neuron is critical for the development of therapeutic treatments for the dysregulation of fluid and electrolyte homeostasis and blood pressure.
The current projects in my laboratory are:
Sexually Dimorphic Oxytocin Receptor Expressing Neurons in the brain
The neurohypophysial hormone oxytocin is known to modulate many aspects of social behaviors, including social recognition, maternal behavior, and pair bonding. Oxytocin influences social behaviors by binding to the oxytocin receptor (OXTR) located in various parts of the brain. In recent years, the oxytocin system in the brain has received tremendous attention as a potential pharmacological target for the treatment of many psychiatric disorders, such as anxiety, autism spectrum disorders, and postpartum depression. Despite the importance, the cellular characterization and connectivity of OXTR expressing neurons in the brain is still largely unknown. We recently discovered a group of estrogen-dependent OXTR neurons that is exclusively present in the hypothalamic anteroventral periventricular nucleus (AVPV) in females, but not in males. The overall long-term objective of our project is to elucidate the behavioral significance and regulatory mechanisms of the OXTR neurons in the AVPV. Because the AVPV is known to regulate parental behavior in a sex-specific manner, we hypothesized that oxytocin exerts parental behavior via OXTR neurons in the AVPV. To address this hypothesis, we will employ a "Designer Receptors Exclusively Activate by Designer Drug" (DREADD)-based approach to specifically manipulate activity of OXTR neurons in the AVPV in vivo and in vitro. Both the stimulatory and inhibitory DREADD will be introduced specifically to OXTR neurons using Cre-recombinase-dependent viral vectors. DREADDs are mutated G-protein coupled receptors that are exclusively activated by the pharmacologically inert ligand clozapine-N-oxide (CNO) at nanomolar potency. Thus, neural activity of the DREADD-expressing OXTR neurons can be manipulated by CNO. Currently, we are examining the effect of inhibition/activation of OXTR neurons in the AVPV by systemic administration of CNO on maternal behavior. Additionally, we are investigating the anatomical and functional connectivity of OXTR neurons in the AVPV using neural tracing techniques combined with electrophysiology and Ca++ imaging. The proposed studies will elucidate the sex-specific oxytocin neural circuitry system that regulate sex-specific social behavior. The findings from this project will provide useful insight into sex-specific pharmacological interventions that treat sex typical psychiatric disorders such as postpartum depression (PPD). This project is funded by the Louisiana Board of Regents and NIMH/NIH R21MH119609.
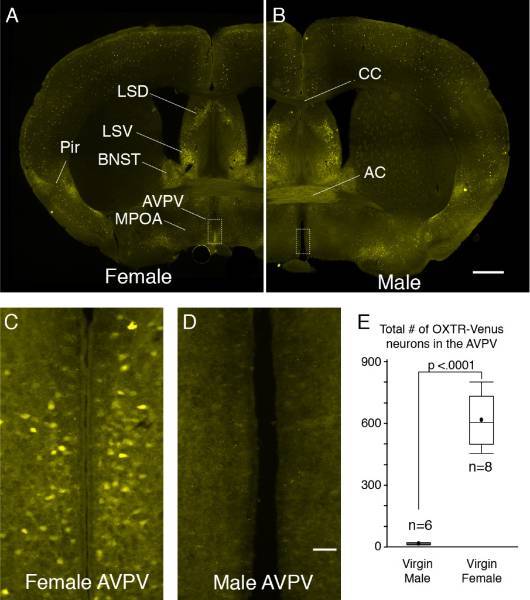
Fig. 3. OXTR-Venus expression in the AVPV are sexually dimorphic. OXTR-Venus expression in a coronal section at the level of the anterior commissure (AC) from a female (A) and a male mouse (B). Unlike transgenic models which use random integration of reporter gene that could end up anywhere in the host genome, this OXTR-Venus mouse line is an OXTR knock-in model in which Venus is inserted into exactly the locus where OXTR is normally located. Therefore, Venus likely achieves natural expression patterns and levels, while ectopic expression less likely occurs. The overall distribution of OXTR-Venus cell was largely comparable to that of OXTR detected by in situ hybridization and oxytocin bindings. Higher power views of the AVPV (C&D) indicated by a rectangle in A and B, respectively. There are no OXTR-Venus neurons in the AVPV in the male. The total number of OXTR-Venus neurons in the AVPV is significantly higher in females than males (E). Scale bar for A is 1 mm and for C is 0.2 mm. Dorsal lateral septum (LSD), ventral lateral septum (LSV), bed nucleus of the stria terminalis (BNST), medial preoptic areas (MPOA), anteroventral periventricular nucleus (AVPV), corpus callosum (CC).
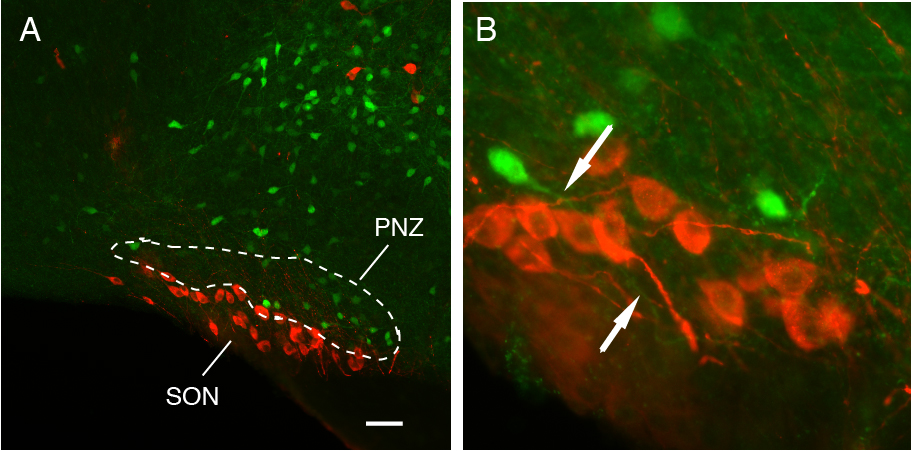
Regulation of Oxytocin neurons by neurons in the Perinuclear Zone
The purpose of this research is to characterize the autoregulatory mechanism of the hypothalamic oxytocin system on the systemic release of oxytocin during reproductive states. The systemic release of oxytocin, essential for birth and normal growth of offspring, is largely regulated by the somato-dendritic release of oxytocin that is elicited by physiological stimuli, such as parturition and suckling. OXTR was found in neurons in the perinuclear zone (PNZ), the area immediately dorsal to the SON. Moreover, the OXTR cells in the PNZ have processes projecting into the SON. These findings strongly suggest that the somato-dendritic release oxytocin on oxytocin neuron is mediated by the OXTR neurons in the PNZ. We are using an integrative multidisciplinary approach that includes electrophysiology combined with optogenetic manipulation of neural activity, neural tracing, immunocytochemistry on transgenic mouse models to explicitly investigate how OXTR neurons in the PNZ regulate oxytocin neurons in the SON. The findings from these studies will provide new information concerning the regulation of oxytocin release and the neural adaptations that are necessary for proper parturition and lactation. NICHD/NIH R21HD097565
Epithelial Sodium Ion Channel (ENaC) in vasopressin Neurons
This project is to investigate the mechanism by ENaC modulate the secretion of the neurohypophysial hormones vasopressin in relate to the development of salt-sensitive hypertension. Our study demonstrated that ENaC is specifically located in vasopressin neurons and mediate a Na+-leak current modulating membrane potential. Thus, ENaC affect the frequency of action potentials in vasopressin neurons. Our recent studies showed that animals that consumed high dietary salt displayed an enhanced ENaC expression, ENaC protein abundance, and ENaC current, which resulted in further depolarization of the basal membrane potential of vasopressin neurons. These findings indicate that high dietary salt intake enhances the expression and activity of ENaC, which in turn, augments synaptic drive during hormonal demand by depolarizing the basal membrane potential closer to the action potential threshold. Activation of ENaC, therefore, is a powerful means to modulate hormone secretion according to physiological demands. Considering the role of VP in the cardiovascular homeostasis, ENaC in vasopressin neurons may have a major role in the regulation of blood pressure. This project is funded by NLHBI/NIH R21 HL093728-01 and R01HL115208-01.
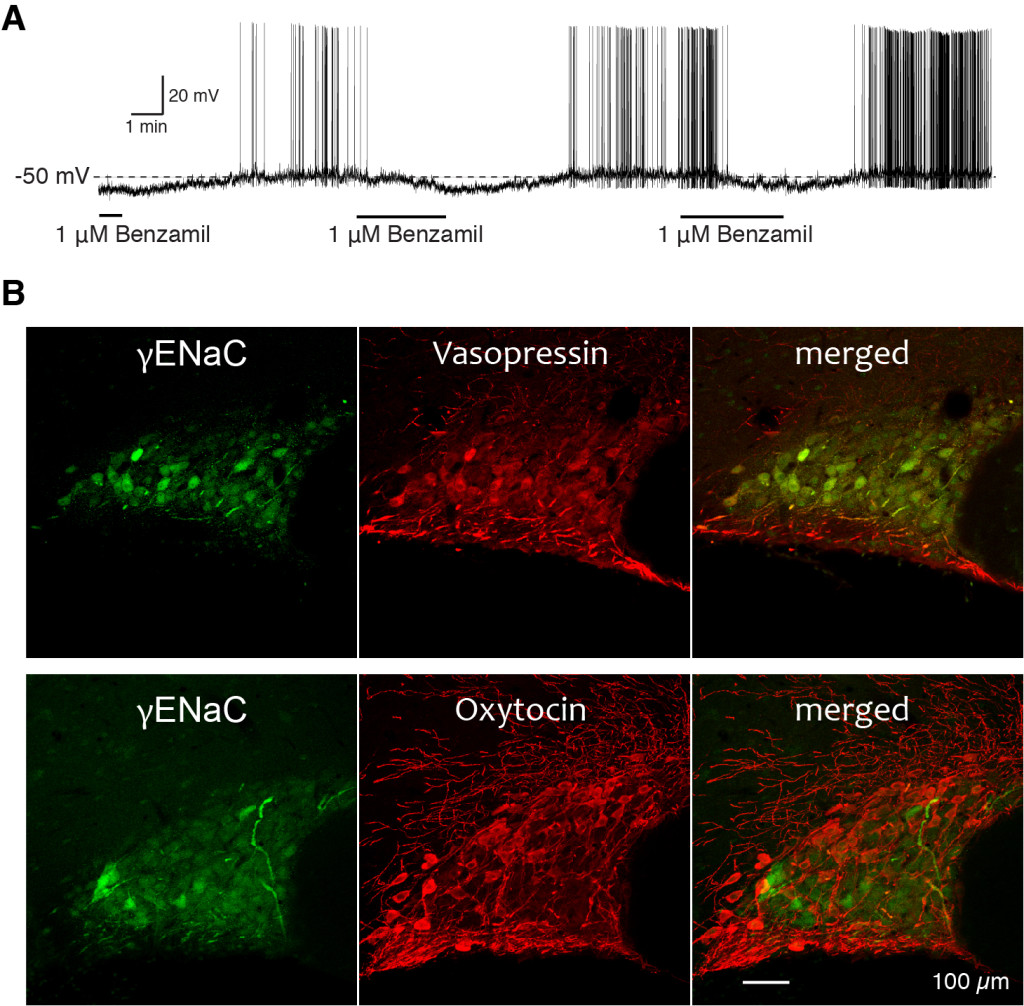
Fig. 4 A. An example of the effect of ENaC blocker, benzamil, on the membrane potential and the firing pattern of vasopressin neuron. The removal of benzamil from the media caused depolarization and firing of the vasopressin neurons. Subsequent bath application of benzamil caused membrane hyperpolarization and cessation of the firing. No current was injected during the recording. B. Immunocytochemical localization of gENaC subunit with vasopressin (top panels) and with oxytocin (bottom panels) in the rat SON. Oxytocin and vasoressin immunoreacitivitis were labeled with red fluorescence dye, while gENaC subunit immunoreactivity was labeled with green fluorescence dye. Note that the merged images reveled that these gENaC immunoreactive neurons are all vasopressin neurons, but not oxytocin neurons.
Alligators are aggressive because of an enlarged medulla oblangata
This project was started, because we wanted to have a neurobiological project that is unique to Louisiana. The ultimate goal of this study is to create the map of alligator brain for comparative vertebrate neuroanatomy. Great difference in specialization of the brain exists between species due to the results of adaptation to very different modes of existence. Thus, studying neuroanatomy of the alligator brain may provide critical information about evolution and adaptation of vertebrate animals. Because nomenclature for specific neural structures is not determined in the alligator brain, we are constructing a stereotaxic atlas of the alligator brain from serial brain section using a stereotaxic apparatus modified specifically for alligator head and a micro CT scan.
Why are alligators so ornery?" To which Bobby Bouche (played by Adam Sandler in movie, The Waterboy) replies "My Mama says that alligators are ornery because they got all them teeth and no toothbrush." The Professor replies back, "Well your Mama is wrong. Alligators are ornery because of their enlarged medulla oblongata!". As a neurobiologist, one of the most frequently asked questions from students and the general public is that if alligators are aggressive because of their enlarged medulla oblongata. The answer is probably not because of their enlarged medulla oblongata, as the involvement of the medulla oblongata in expression of aggressive behaviors has not been found. But, do we know if their medulla oblongata is relatively larger than other vertebrate animals? We actually don't know that.
To this point, we identified several brain areas according to the presence of specific
neurotransmitter/neuromodulator using molecular techniques. One of the neurotransmitters
we located in the brain is Neuropeptide Y (NPY). NPY is produced in various locations
especially high in the hypothalamus. The hypothalamic NPY is thought to involve in
several fundamental function of animal, such as food intake and storage of energy,
and circadian rhythm. We also localized Gonadotropin-Releasing Hormone (GnRH) synthesizing
neurons in the hypothalamus. GnRH plays a pivotal role in reproductive physiology
and behavior of animal. 
Alligator brain
