Research Interests
Research in the Kartika Group focuses on the development of new organic synthetic reactions that leverage discoveries in novel chemical reactivitities to construct complex molecular scaffolds with potential values for biological, pharmaceutical, and industrial applications.
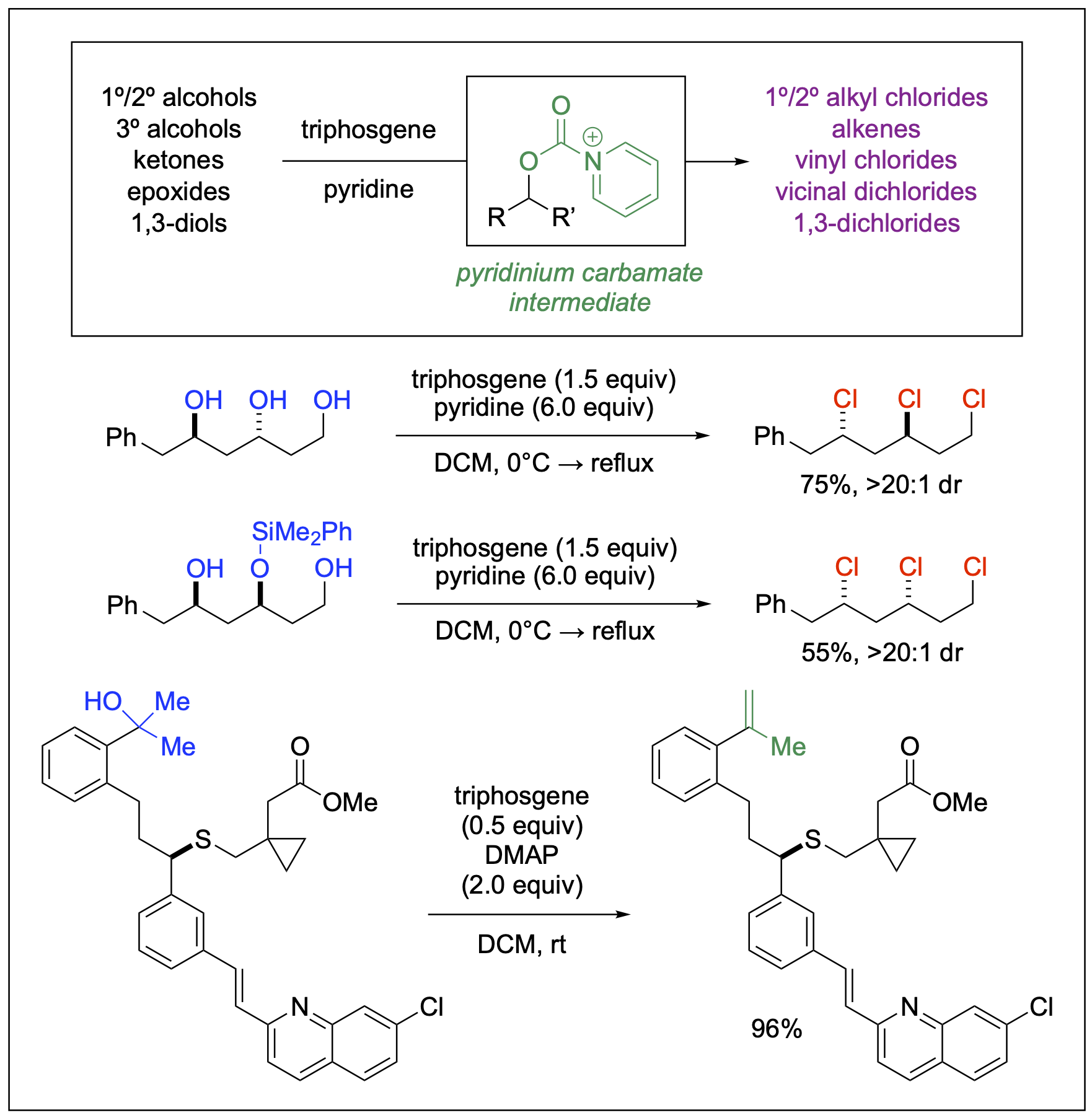
Deoxychlorination Reactions with Triphosgene and Nitrogen Bases
Organochlorines play a significant role in organic chemistry, as demonstrated by their occurrence in chlorine-containing natural products and various synthetic small molecules that are crucial for industrial, agricultural, and pharmaceutical applications. They also function as important precursors in organic reactions and have been utilized in a wide range of synthetic transformations. Given their extensive utility, the development of reactions to form carbon-chlorine bonds remains an important area of research. Our contribution to this field features the use of triphosgene and nitrogen bases, particularly pyridine, to facilitate deoxychlorination reactions of readily available substrates, such as alcohols, ketones, and epoxides. This approach yields a diverse array of organochlorine products, including alkyl chlorides, vinyl chlorides, and vicinal dichlorides. Noteworthy transformations enabled by our chemistries include the diastereoselective global deoxychlorination of 1,3,5-triols and the chemoselective dehydration of tertiary alcohols using triphosgene and DMAP as mild reagents. The advantages of our technology are highlighted by its broad tolerability and procedural simplicity, as it does not necessitate inert or anhydrous conditions. Triphosgene is a stable, non-hygroscopic crystalline material at room temperature, which makes it safer to handle in the laboratory compared to the hazardous phosgene gas. Furthermore, the crude materials produced from our reactions often demonstrate high product purity, thereby eliminating the need for column chromatography.
Relevant Publications
- Ganiu, M. O.; Cleveland, A. H.; Paul, J. L.; Kartika, R. “Triphosgene and DMAP as Mild Reagents for Chemoselective Dehydration of Tertiary Alcohols.” Org. Lett. 2019, 21, 5611. DOI: 10.1021/acs.orglett.9b01959.
- Cleveland, A. H.; Fronczek, F. R.; Kartika, R. "Synthesis of Vicinal Dichlorides via Activation of Aliphatic Terminal Epoxides with Triphosgene and Pyridine." J. Org. Chem. 2018, 83, 3367. DOI: 10.1021/acs.joc.7b03197. (Highlighted in Organic Chemistry Portal – ID: J42-Y2018)
- Villalpando, A.; Saputra, M. A.; Tugwell, T. H.; Kartika, R. "Triphosgene-Pyridine Mediated Stereoselective Chlorination of Acyclic Aliphatic 1,3-Diols." Chem. Commun. 2015, 51, 15075. DOI: 10.1039/C5CC06365E.
- Saputra, M. A.; Ngo, Ly; Kartika, R. "Synthesis of Vinyl Chlorides via Triphosgene-Pyridine Activation of Ketones." J. Org. Chem. 2015, 80, 8815. DOI: 10.1021/acs.joc.5b01137.
- Villalpando, A.; Ayala, C. E.; Watson, C. B.; Kartika, R. "Triphosgene-Amine Base Promoted Chlorination of Unactivated Aliphatic Alcohols." J. Org. Chem. 2013, 78, 3989. DOI: 10.1021/jo400341n. (Highlighted in Organic Chemistry Portal – ID: J42-Y2013-1010)
- Ayala, C. E.; Villalpando, A.; Nguyen, A. L.; McCandless, G. T.; Kartika, R. "Chlorination of Aliphatic Primary Alcohols via Triphosgene-Triethylamine Activation." Org. Lett. 2012, 14, 3676. DOI: 10.1021/ol301520d. (Highlighted in Organic Chemistry Portal – ID: J54-Y2012-2320)
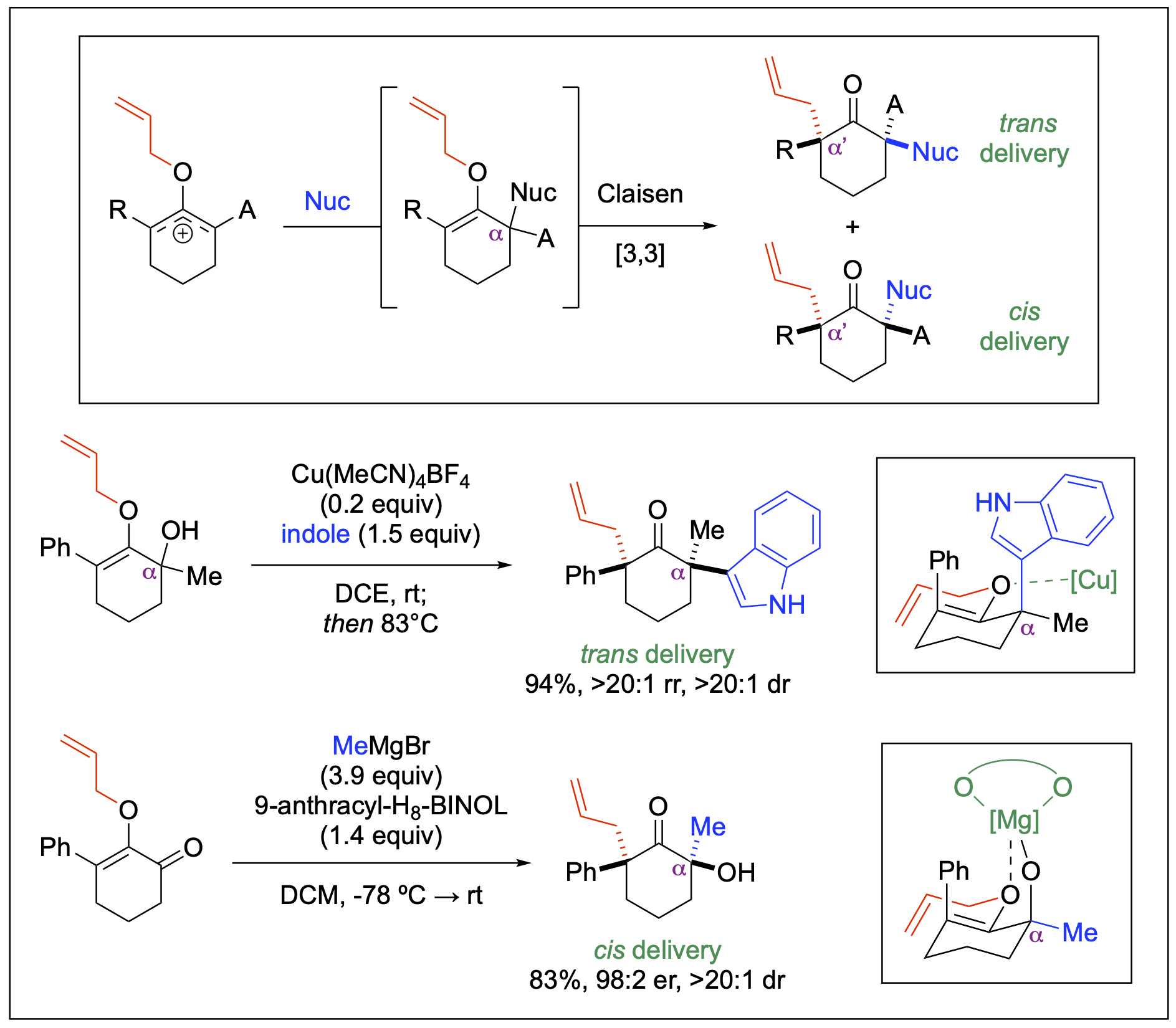
Quaternary Centers
Quaternary centers represent significant structural motifs in drug discovery; however, their broader applications in medicinal chemistry have been constrained by the considerable challenges associated with the synthesis of these sterically congested systems. Our work in this area features stereoselective syntheses of α-quaternary centers, highlighting new strategies that utilize the opposing α-stereocenter to leverage the Claisen Rearrangement to provide direct access to synthetically challenging molecular structures, including fully substituted ketones at the α,α'-positions. Additionally, we have successfully utilized our chemistry to functionalize bioactive molecules, such as formestane.
Relevant Publications
- Philkhana, S. C.; Malone, J. A.; Armendariz-Gonzales, E.; Saputra, E.; Fronczek; F. R.; Kartika, R. "Synthesis of bis-Quaternary Centers at the α-Positions of Cyclohexanones via Copper(I)-Catalyzed Claisen Rearrangement: The Substituent Effect from the Opposing α-Quaternary Center on Diastereoselectivity." Tetrahedron 2024, Manuscript Accepted.
- Badmus, F. O.; Thombal, R. S.; Philkhana, S. C.; Malone, J. A.; Bailey, C. E.; Armendariz-Gonzales, E.; Mureka, E. W.; Locicero, C. M.; Fronczek; F. R.; Kartika, R. “Directing the Stereoselectivity of the Claisen Rearrangement to Form Cyclic Ketones with Full Substitution at the α-Positions.” Org. Lett. 2023, 25, 7622. DOI: 10.1021/acs.orglett.3c02752.
- Malone, J. A.; Philkhana, S. C.; Stepherson, J. R.; Badmus, F. O.; Fronczek; F. R.; Kartika, R. “Copper(I)-Catalyzed Synthesis of Unsymmetrical All-Carbon bis-Quaternary Stereocenters at the Opposing α-Carbons of Cyclohexanones.” Org. Lett. 2022, 24, 4810. DOI: 10.1021/acs.orglett.2c01890.
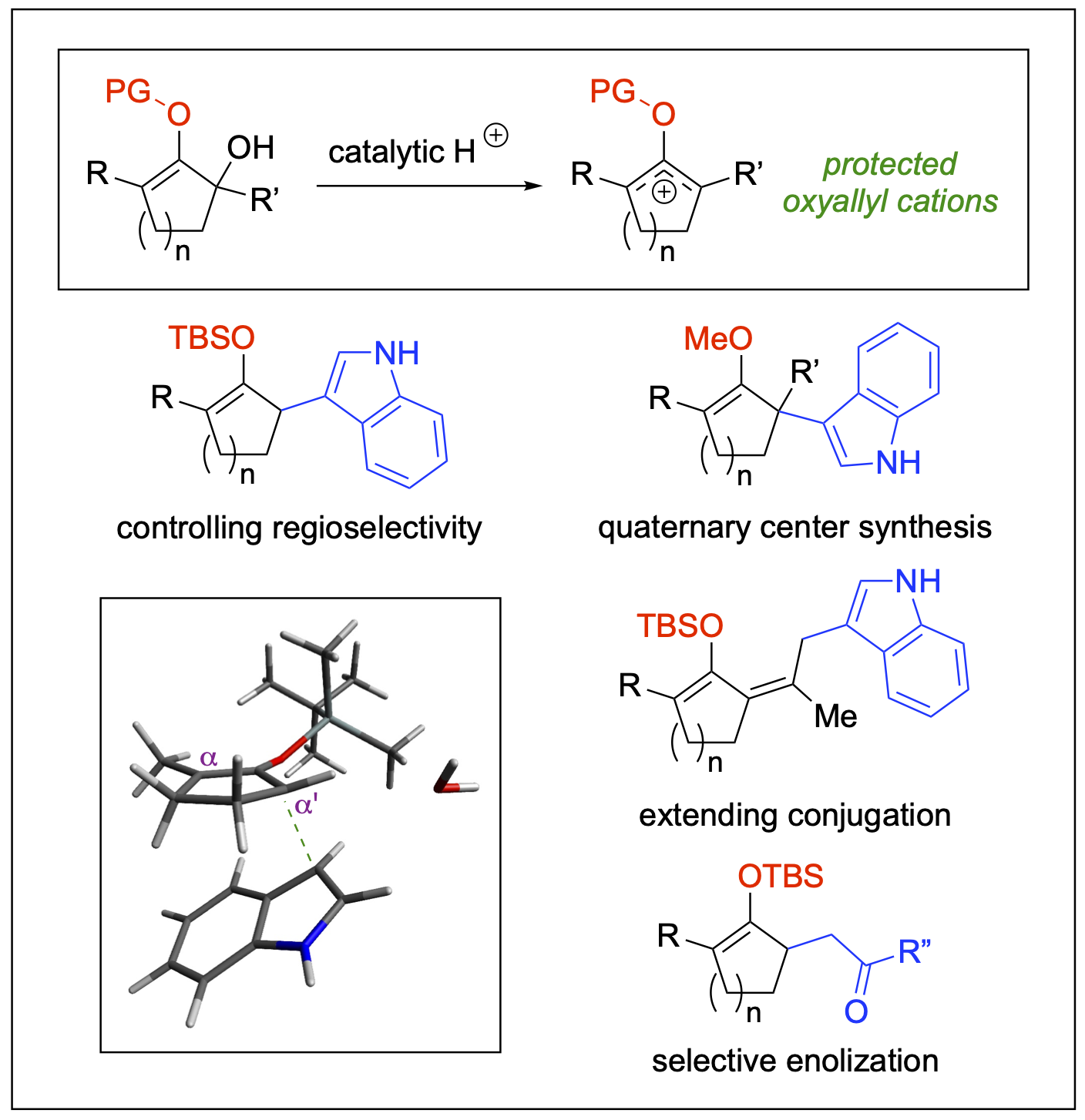
Unsymmetrical Protected Oxyallyl Cations
Oxyallyl cations are structurally unique reactive intermediates. Due to the distribution of their electrophilic character over three carbon atoms, these species are universally regarded as the fleeting intermediates in the Nazarov-type cyclization and various cycloaddition reactions. Our research in this area showcases new approach to harness unusual reactivity of unsymmetrical oxyallyl cations toward regioselective addition of nucleophiles. By introducing appropriate “protecting” groups in the oxygen atom center, unsymmetrical oxyallyl cations could be generated under mild catalytic conditions and leveraged for the synthesis of diverse molecular scaffolds of biological and pharmaceutical relevance.
Relevant Publications
- Bresnahan, C.; Taylor-Edinbyrd, K. A.; Cleveland, A. H.; Malone, J. A.; Dange, N. S.; Millet, A.; Kumar, R.; Kartika, R. “Mechanistic Perspectives into the Regioselective Indole Addition to Unsymmetrical Silyloxyallyl Cations.” J. Org. Chem. 2019, 84, 7166. DOI: 10.1021/acs.joc.9b00853.
- Malone, J. A.; Van Houten, J. P.; Ganiu, M. O.; Nepal, B.; Kartika, R. "Brønsted Acid Catalyzed Synthesis of Functionalized 1,4- and 1,6-Dicarbonyl Monosilylenol Ethers under Operationally Practical Conditions." J. Org. Chem. 2017, 82, 10659. DOI: 10.1021/acs.joc.7b01687.
- Malone, J. A.; Cleveland, A. H.; Fronczek, F. R.; Kartika, R. "Effects of Solvent and Residual Water on Enhancing the Reactivity of 6-Membered Silyloxyallyl Cations towards Nucleophilic Addition." Org. Lett. 2016, 18, 4408. DOI: 10.1021/acs.orglett.6b02194.
- Ayala, C. E.; Dange, N. S.; Stepherson, J. R.; Henry, J. L.; Fronczek, F. R.; Kartika, R. "Functionalization of Silyldienol Ethers at the γ-Position via 2-Silyloxypentadienyl Cations." Org. Lett. 2016, 18, 1084. DOI: 10.1021/acs.orglett.6b00196.
- Dange, N. S.; Stepherson, J. R.; Ayala, C. A.; Fronczek, F. R.; Kartika, R. "Cooperative Benzylic-Oxyallylic Stabilized Cations: Regioselective Construction of Alpha-Quaternary Centers in Ketone-Derived Compounds." Chem. Sci. 2015, 6, 6312. DOI: 10.1039/C5SC01914A.
- Ayala, C. A.; Dange, N. S.; Fronczek; F. R.; Kartika, R. "Brønsted Acid Catalyzed α'-Functionalization of Silylenol Ethers with Indoles." Angew. Chem. Int. Ed. 2015, 54, 4641. DOI: 10.1002/anie.201409758.
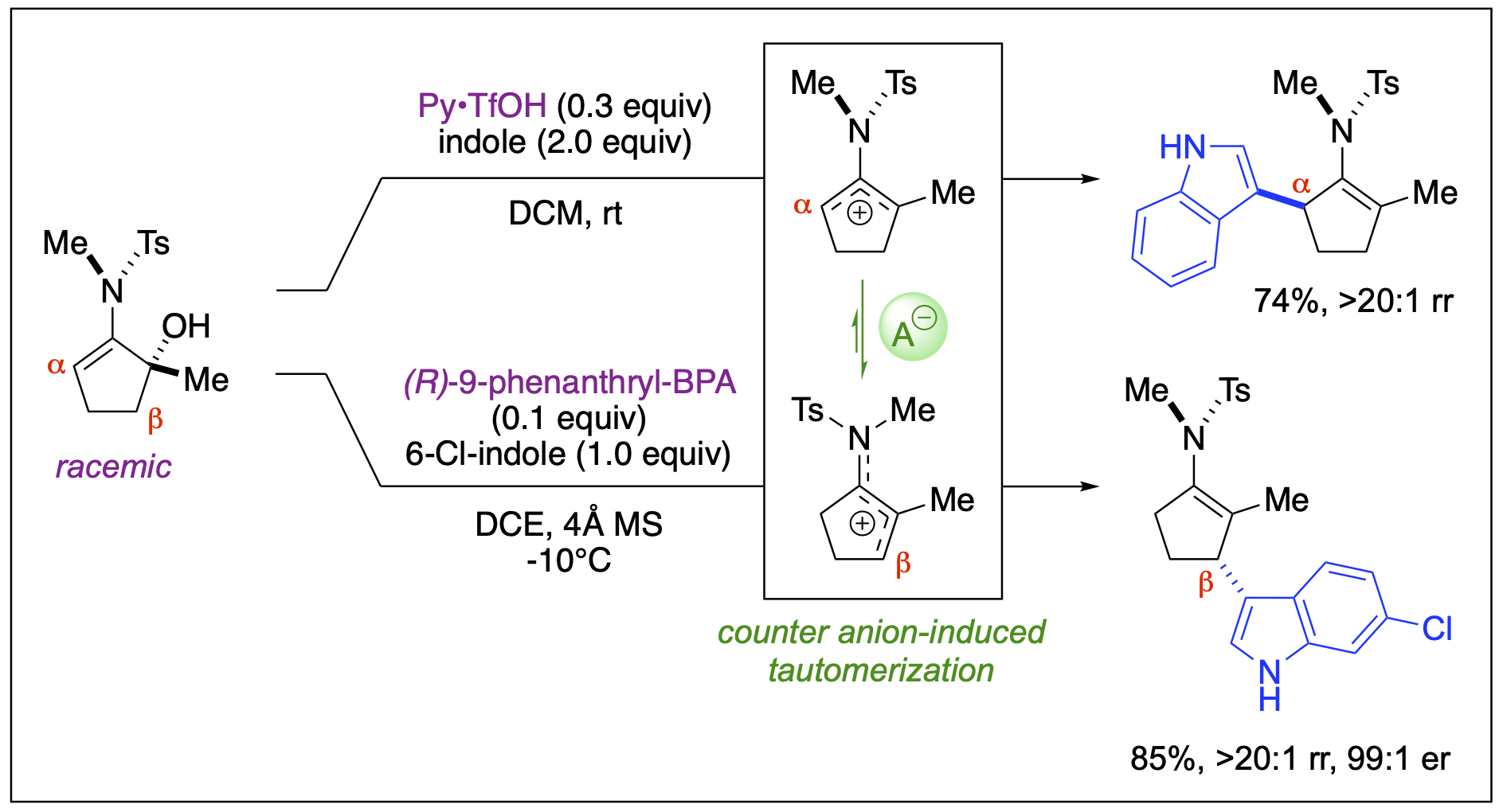
Unsymmetrical Amidoallyl Cations
We have demonstrated new synthetic reactions involving unsymmetrical amidoallyl cations for the synthesis of nitrogen-containing molecular entities. By stabilizing the reactivity of these electrophilic species, we developed catalytic Brønsted acid conditions that effectively facilitate intermolecular nucleophilic addition. Additionally, our findings revealed the unusual reactivity of unsymmetrical 2-amidoallyl cations toward tautomerization induced by counter anions, thereby allowing for regioselective functionalization at either the α- or β-carbon positions by altering the Brønsted acid catalysts.
Relevant Publications
- Saputra, M. A.; Nepal, B.; Dange, N. S.; Du, P; Fronczek, F. R.; Kumar, R.; Kartika, R. "Enantioselective Functionalization of Enamides at the β‐Carbon Center with Indoles." Angew. Chem. Int. Ed. 2018, 57, 15558. DOI: 10.1002/anie.201808764.
- Saputra, M. A.; Dange, N. S.; Cleveland, A. H.; Malone, J. A.; Fronczek, F. R.; Kartika, R. "Regioselective Functionalization of Enamides at the α-Carbon via Unsymmetrical 2-Amidoallyl Cations." Org. Lett. 2017, 19, 2414. DOI: 10.1021/acs.orglett.7b00962.
Heterocycle Synthesis
Our research group has a longstanding interest in heterocyclic chemistry, largely due to the prevalence of heterocycles in drug discovery. Heterocycles are structural units often found in pharmaceutical compounds. Owing to their distinctive physicochemical and electronic properties, many heterocycles have an increased tendency to engage in intermolecular interactions with biological targets, such as proteins, DNA, and antibodies. Our research focuses on cascade reactions under catalytic conditions that leverage the reactivity of silyloxyallyl cations as key intermediates.
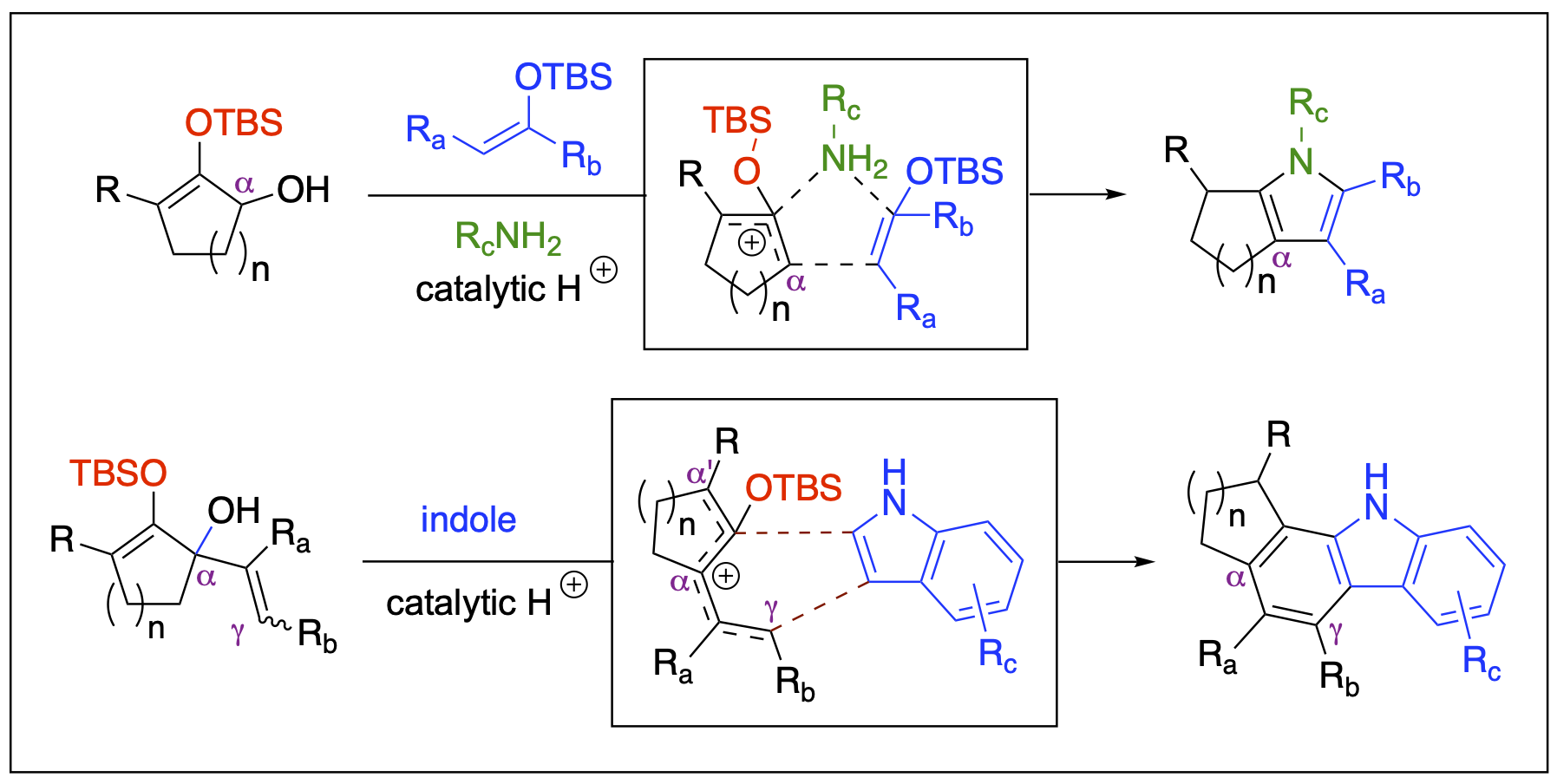
Relevant Publications
- Badmus, F.; Malone, J. A.; Fronczek, F. R.; Kartika, R. “Expedient Synthesis of Functionalized Tetrahydrobenzofuran via Cascade Cycloaddition Involving Silyloxyallyl Cation Intermediate.” Chem. Commun., 2020, 56, 5034. DOI: 10.1039/D0CC01796E. Correction: Chem. Commun., 2020, 56, 6154. DOI: 10.1039/D0CC90228D.
- Malone, J. A.; Toussel, C. E.; Fronczek, F. R.; Kartika, R. “Brønsted Acid-Catalyzed Formal [2 + 2 + 1] Annulation for the Modular Synthesis of Tetrahydroindoles and Tetrahydrocyclopenta[b]pyrroles.” Org. Lett. 2019, 21, 3610. DOI: 10.1021/acs.orglett.9b01032.
- Stepherson, J. R.; Ayala, C. E.; Tugwell, T. H.; Henry, J. L.; Fronczek, F. R.; Kartika, R. "A New Approach to Carbazole Annulation via Cascade Nucleophilic Addition – Cyclization Involving 2-Silyloxypentadienyl Cation." Org. Lett. 2016, 18, 3002. DOI: 10.1021/acs.orglett.6b01376.
Funding Agencies
We gratefully acknowledge the generous financial support for our research from the following funding agencies:
- National Science Foundation (NSF)
- National Institute of General Medical Sciences (NIGMS)
- Louisiana Board of Regents
- Louisiana State University