Publications
As the Corresponding Author
- Armendariz-Gonzalez, E.; Saputra, A.; Mureka, E. W.; Locicero, C. M.; Womble, G. L.; Tan, G.; Watson, A. A.; Fronczek, F. R.; Kartika, R. "Cascade Grignard Addition – Propargyl Claisen Rearrangement for the Stereoselective Synthesis of α-Allene Quaternary Centers in Cyclohexanones." Manuscript Submitted.
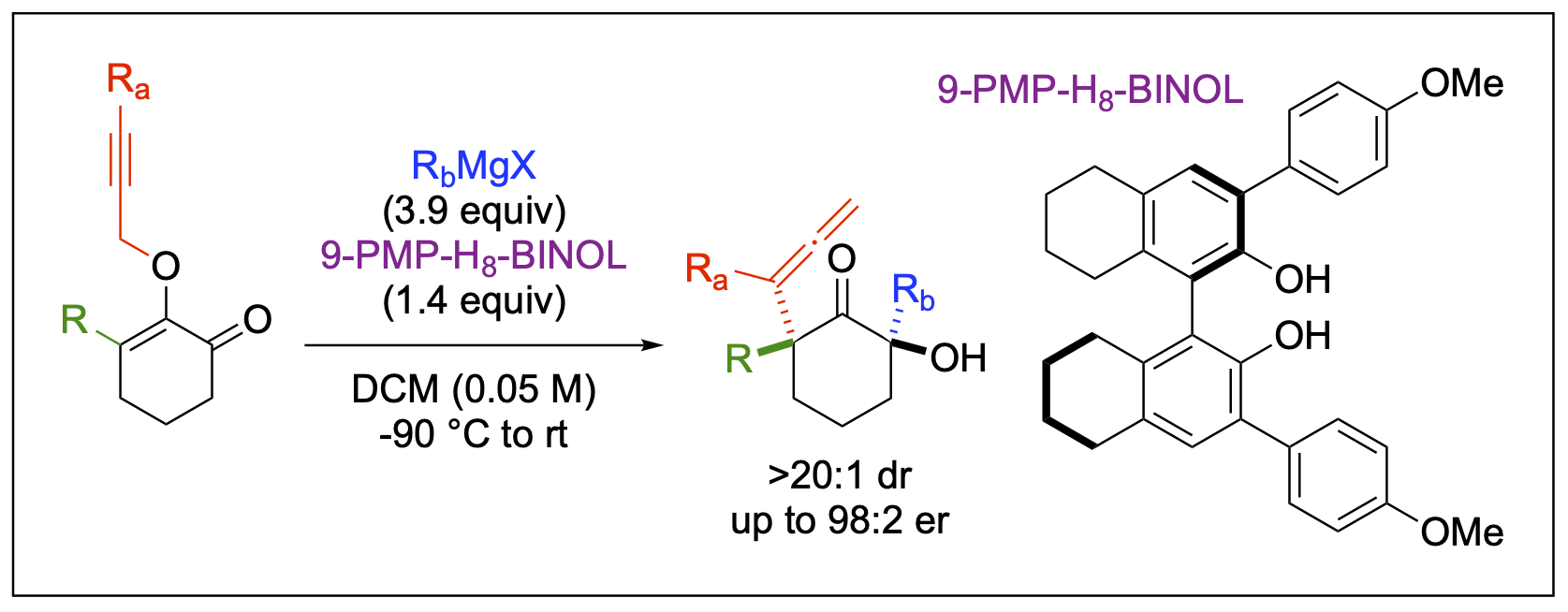
- Philkhana, S. C.; Malone, J. A.; Armendariz-Gonzales, E.; Saputra, E.; Fronczek; F. R.; Kartika, R. "Synthesis of bis-Quaternary Centers at the α-Positions of Cyclohexanones via Copper(I)-Catalyzed Claisen Rearrangement: The Substituent Effect from the Opposing α-Quaternary Center on Diastereoselectivity." Tetrahedron 2025, 172, 134415. DOI: 10.1016/j.tet.2024.134415.
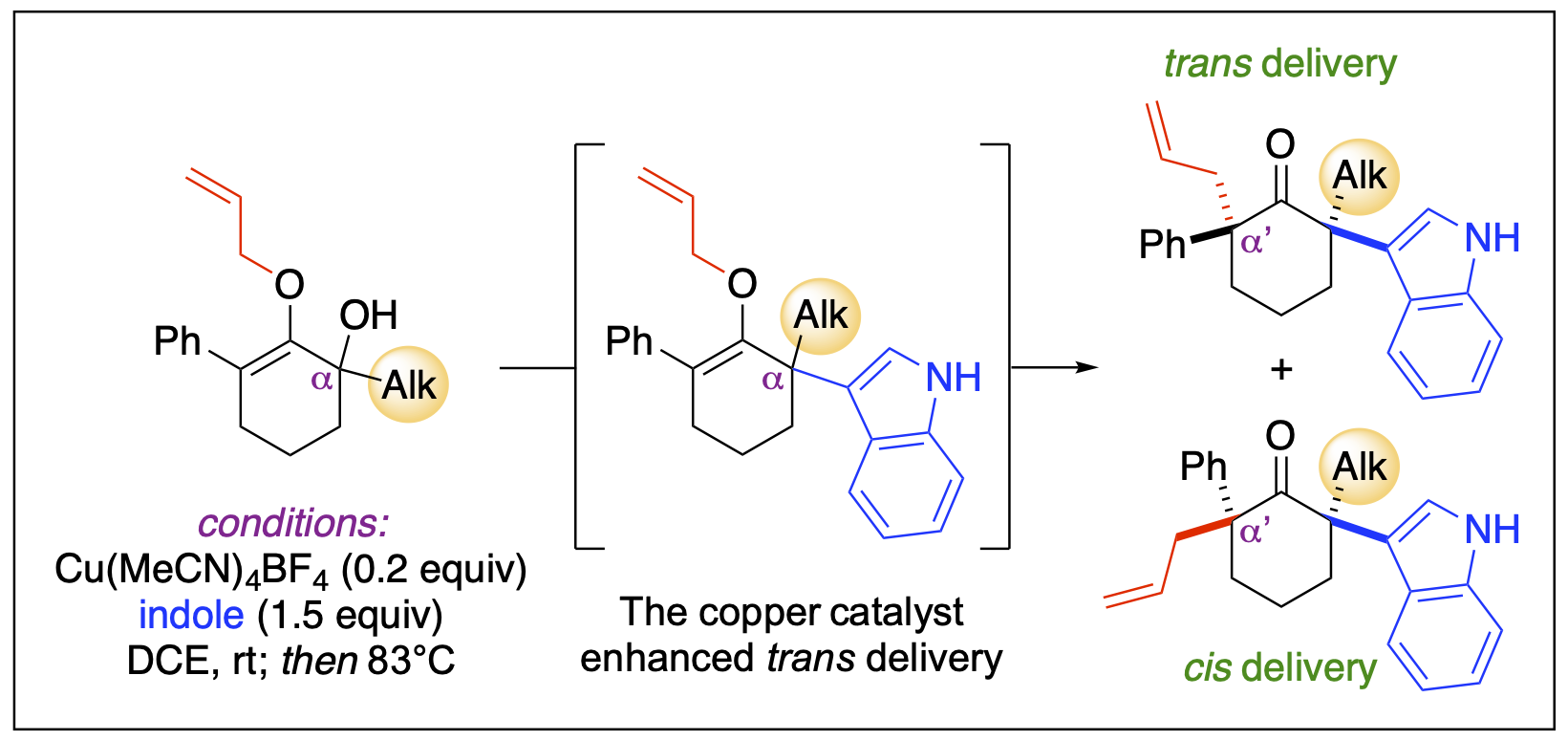
- Badmus, F. O.; Thombal, R. S.; Philkhana, S. C.; Malone, J. A.; Bailey, C. E.; Armendariz-Gonzales, E.; Mureka, E. W.; Locicero, C. M.; Fronczek; F. R.; Kartika, R. “Directing the Stereoselectivity of the Claisen Rearrangement to Form Cyclic Ketones with Full Substitution at the α-Positions.” Org. Lett. 2023, 25, 7622. DOI: 10.1021/acs.orglett.3c02752.
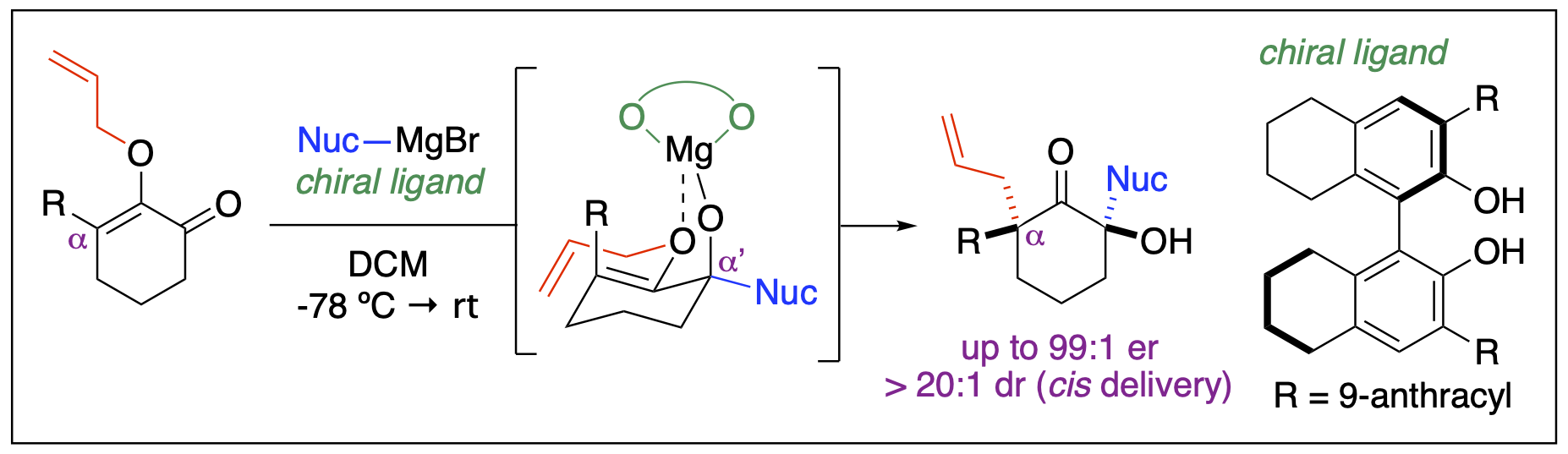
- Malone, J. A.; Philkhana, S. C.; Stepherson, J. R.; Badmus, F. O.; Fronczek; F. R.; Kartika, R. “Copper(I)-Catalyzed Synthesis of Unsymmetrical All-Carbon bis-Quaternary Stereocenters at the Opposing α-Carbons of Cyclohexanones.” Org. Lett. 2022, 24, 4810. DOI: 10.1021/acs.orglett.2c01890.
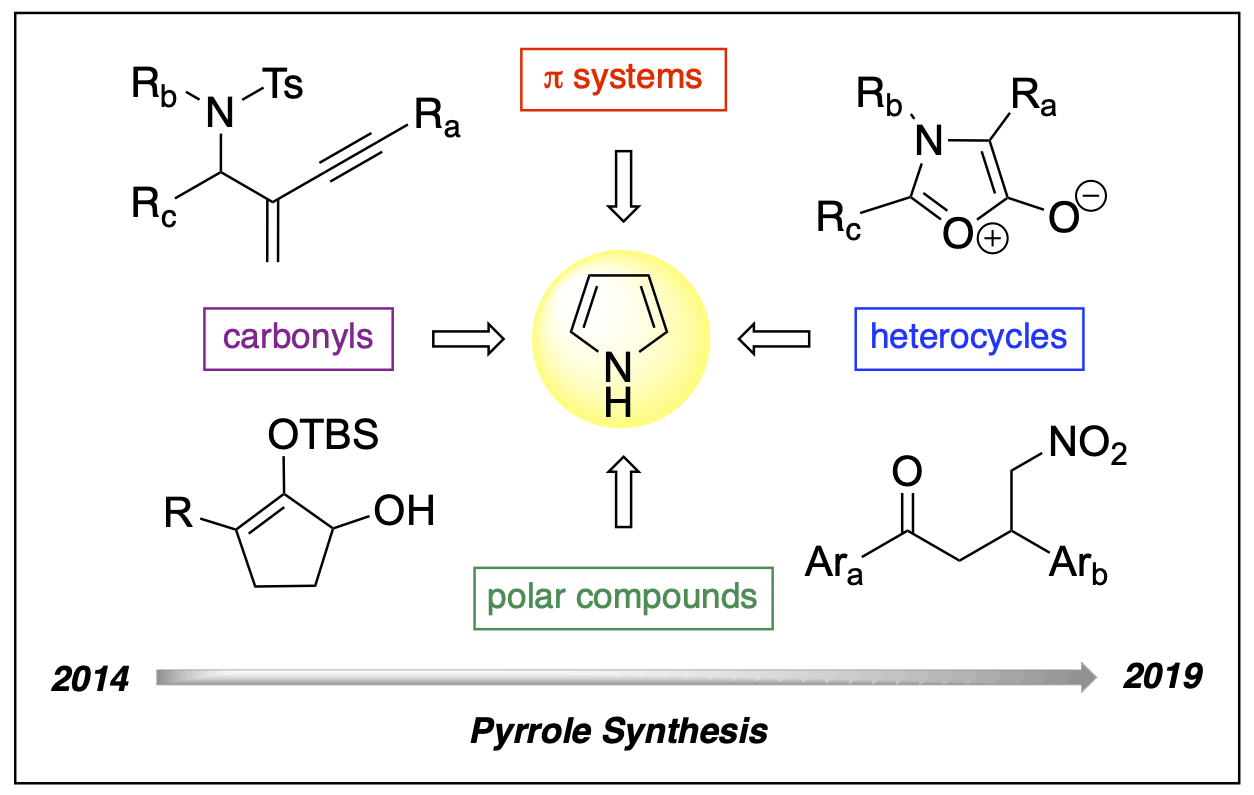
- Philkhana, S. C.; Badmus, F.; Dos Reis, I. C.; Kartika, R. “Recent Advancement of Pyrrole Synthesis.” Synthesis, 2021, 53, 1531. DOI: 10.1055/s-0040-1706713.
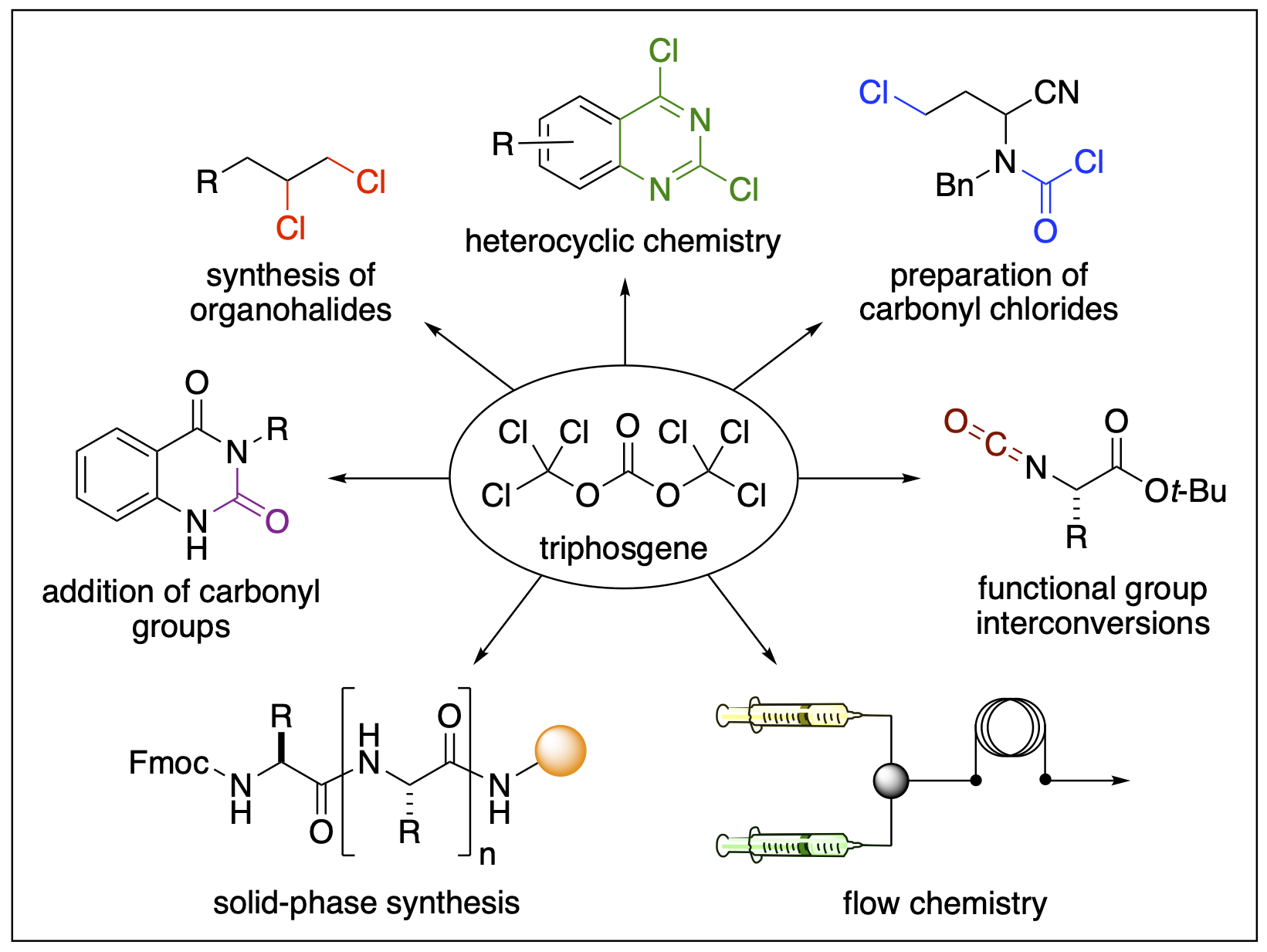
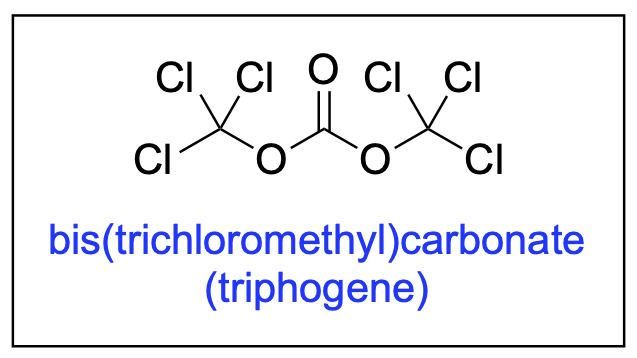
- Ganiu, M. O.; Nepal, B.; Van Houten, J. P.; Kartika, R. “A Decade Review of Triphosgene and Its Applications in Organic Reactions." Tetrahedron, 2020, 76, 131553. DOI: 10.1016/j.tet.2020.131553.
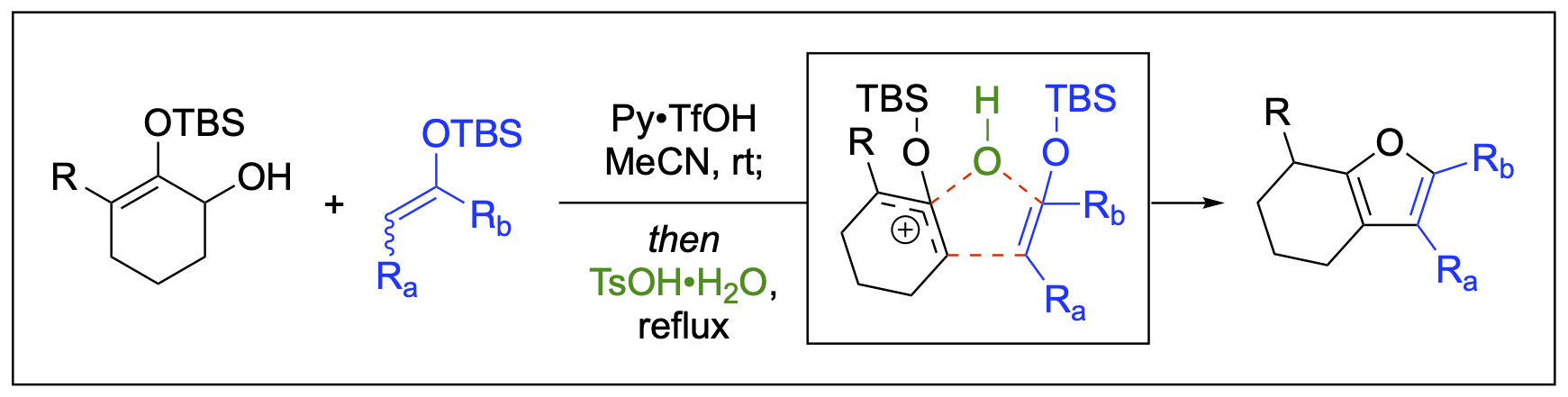
- Badmus, F.; Malone, J. A.; Fronczek, F. R.; Kartika, R. “Expedient Synthesis of Functionalized Tetrahydrobenzofuran via Cascade Cycloaddition Involving Silyloxyallyl Cation Intermediate.” Chem. Commun., 2020, 56, 5034. DOI: 10.1039/D0CC01796E. Correction: Chem. Commun., 2020, 56, 6154. DOI: 10.1039/D0CC90228D.
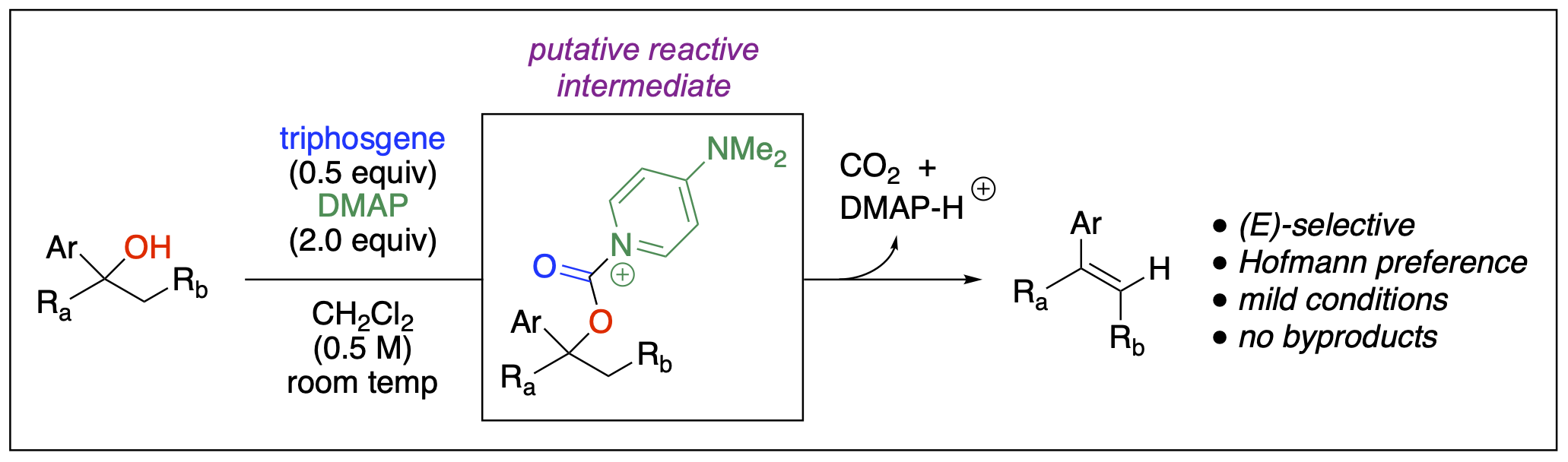
- Ganiu, M. O.; Cleveland, A. H.; Paul, J. L.; Kartika, R. “Triphosgene and DMAP as Mild Reagents for Chemoselective Dehydration of Tertiary Alcohols.” Org. Lett. 2019, 21, 5611. DOI: 10.1021/acs.orglett.9b01959.
- Malone, J. A.; Toussel, C. E.; Fronczek, F. R.; Kartika, R. “Brønsted Acid-Catalyzed Formal [2 + 2 + 1] Annulation for the Modular Synthesis of Tetrahydroindoles and Tetrahydrocyclopenta[b]pyrroles.” Org. Lett. 2019, 21, 3610. DOI: 10.1021/acs.orglett.9b01032.
![a reaction scheme to represent brønsted acid-catalyzed formal [2+2+1] annulation for the modular synthesis of tetrahydroindoles and tetrahydrocyclopenta[b]pyrroles](/rkartika/images/reaction-2019-ol-1-300.png)
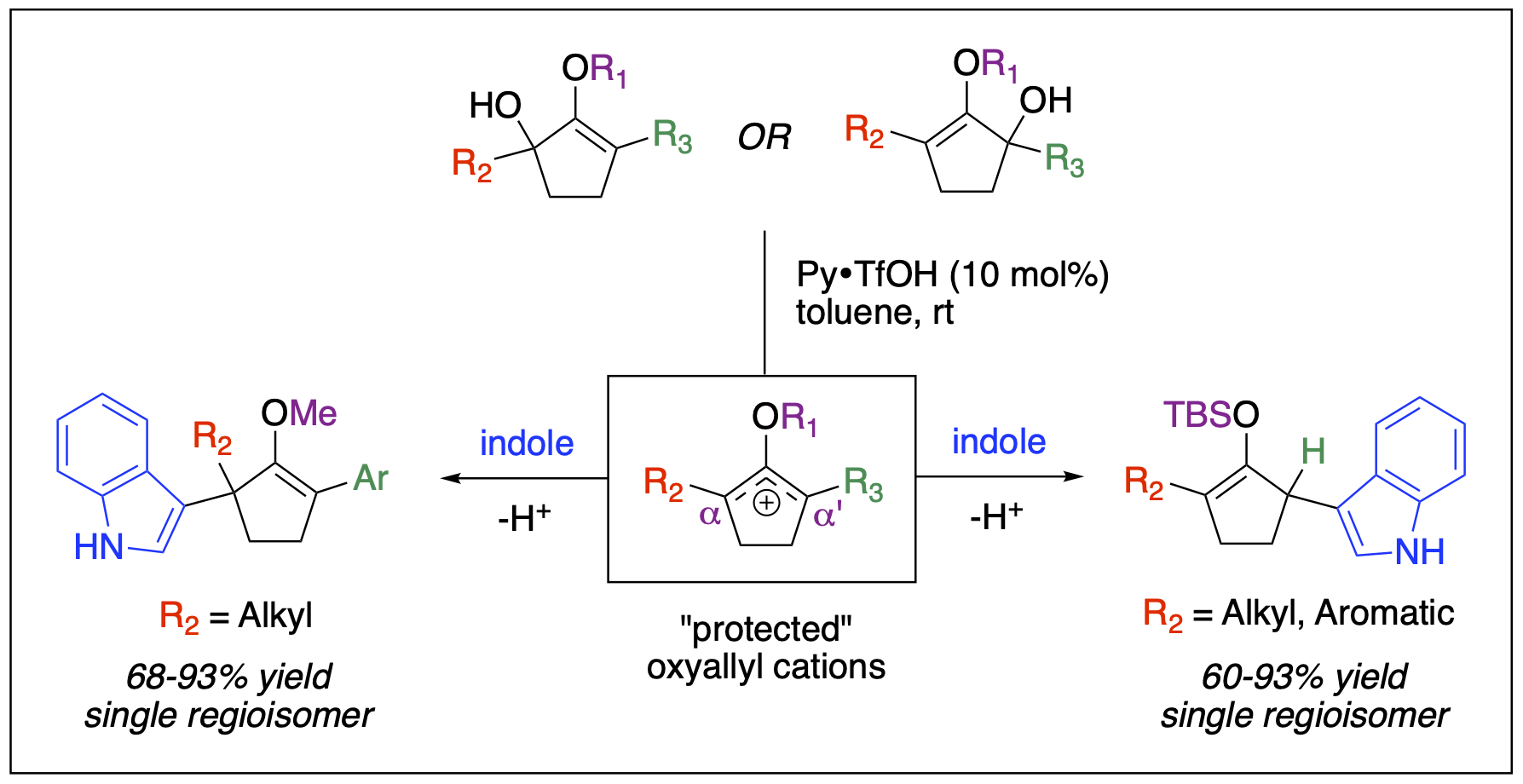
- Bresnahan, C.; Taylor-Edinbyrd, K. A.; Cleveland, A. H.; Malone, J. A.; Dange, N. S.; Millet, A.; Kumar, R.; Kartika, R. “Mechanistic Perspectives into the Regioselective Indole Addition to Unsymmetrical Silyloxyallyl Cations.” J. Org. Chem. 2019, 84, 7166. DOI: 10.1021/acs.joc.9b00853.
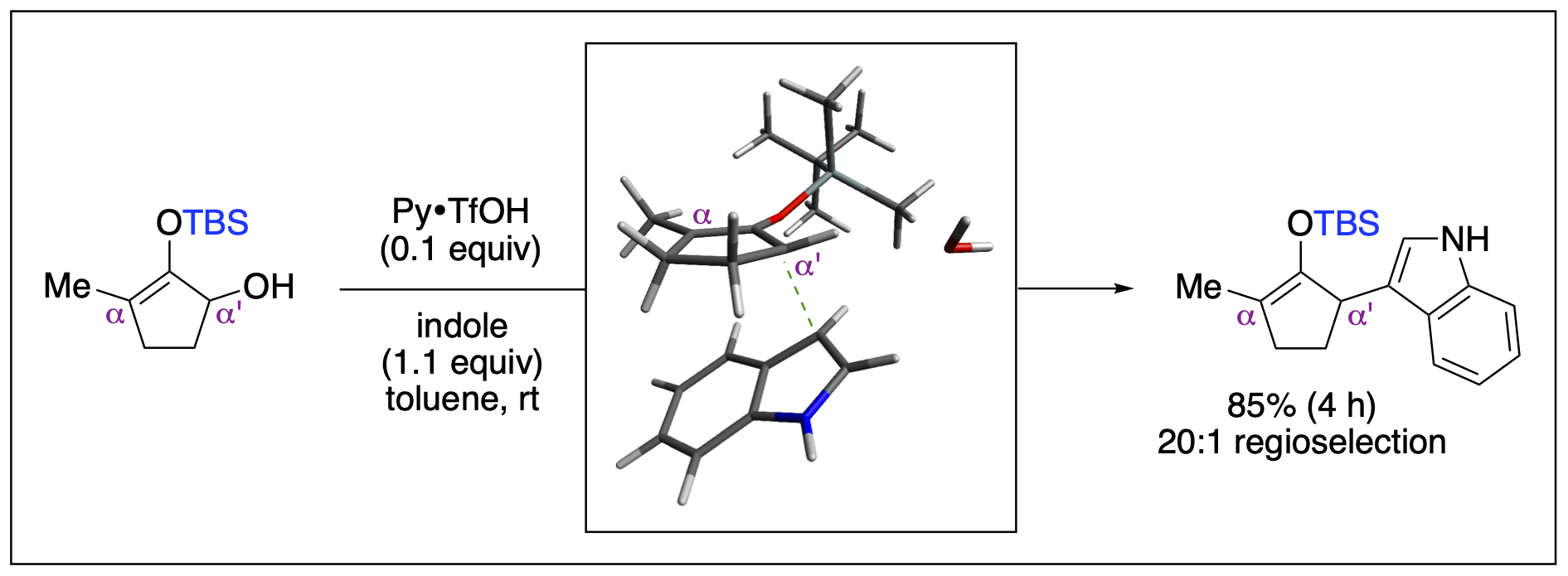
- Saputra, M. A.; Nepal, B.; Dange, N. S.; Du, P; Fronczek, F. R.; Kumar, R.; Kartika, R. "Enantioselective Functionalization of Enamides at the β‐Carbon Center with Indoles." Angew. Chem. Int. Ed. 2018, 57, 15558. DOI: 10.1002/anie.201808764.
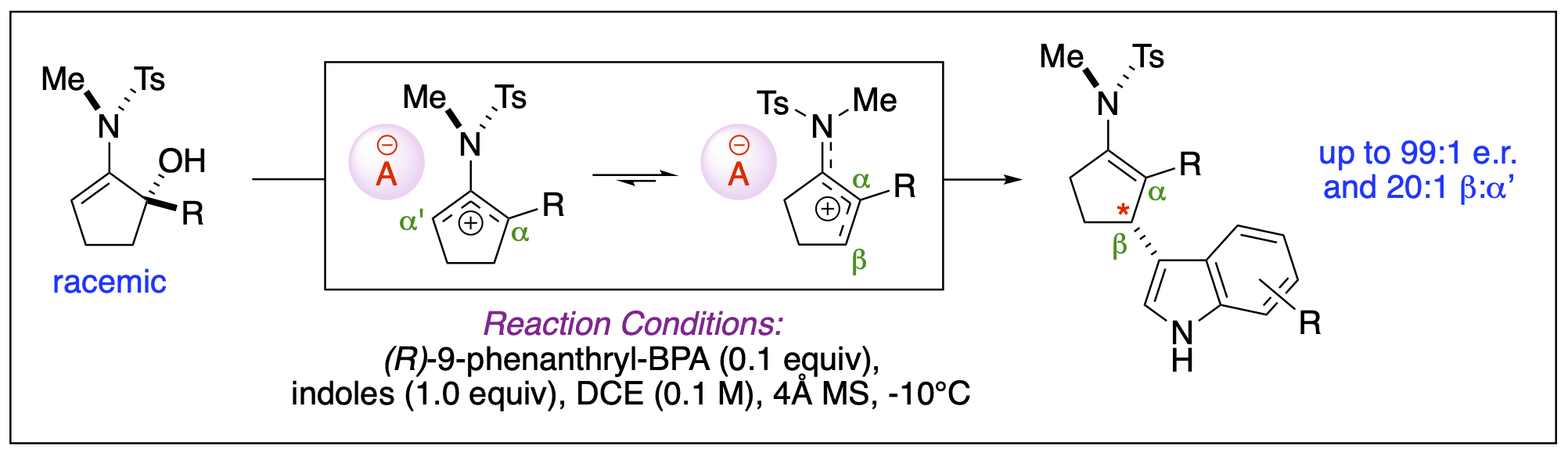
- Cleveland, A. H.; Fronczek, F. R.; Kartika, R. "Synthesis of Vicinal Dichlorides via Activation of Aliphatic Terminal Epoxides with Triphosgene and Pyridine." J. Org. Chem. 2018, 83, 3367. DOI: 10.1021/acs.joc.7b03197. (Highlighted in Organic Chemistry Portal – ID: J42-Y2018)
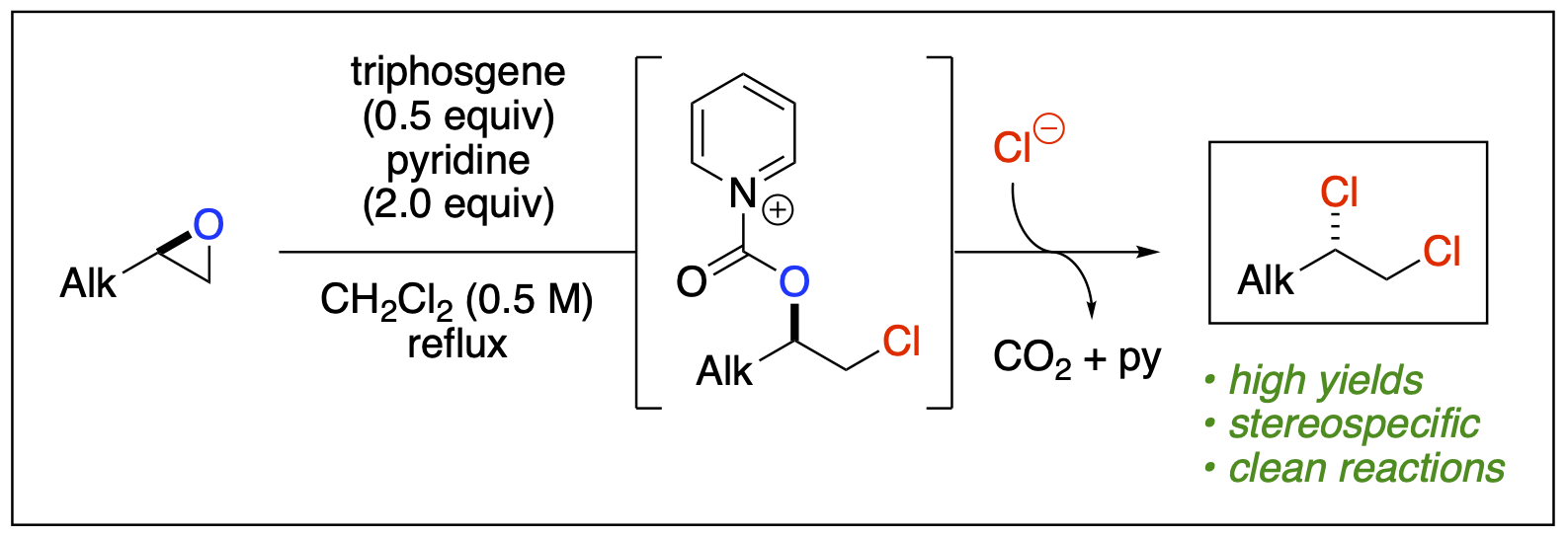
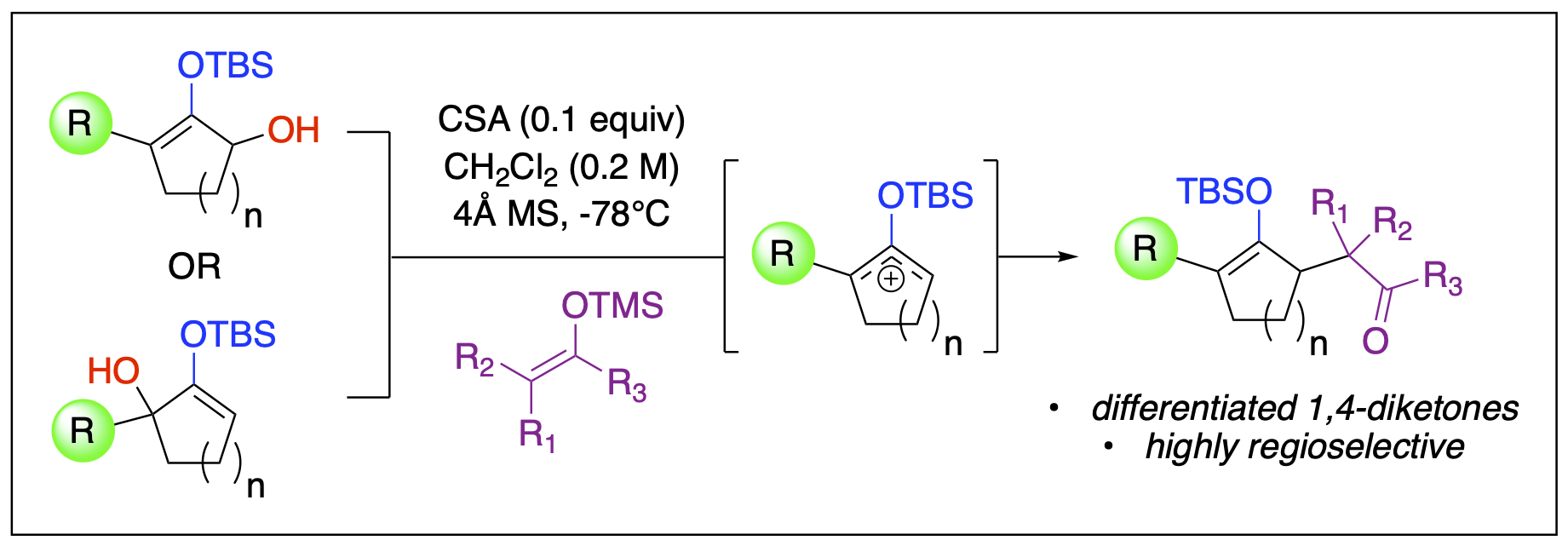
- Malone, J. A.; Van Houten, J. P.; Ganiu, M. O.; Nepal, B.; Kartika, R. "Brønsted Acid Catalyzed Synthesis of Functionalized 1,4- and 1,6-Dicarbonyl Monosilylenol Ethers under Operationally Practical Conditions." J. Org. Chem. 2017, 82, 10659. DOI: 10.1021/acs.joc.7b01687.
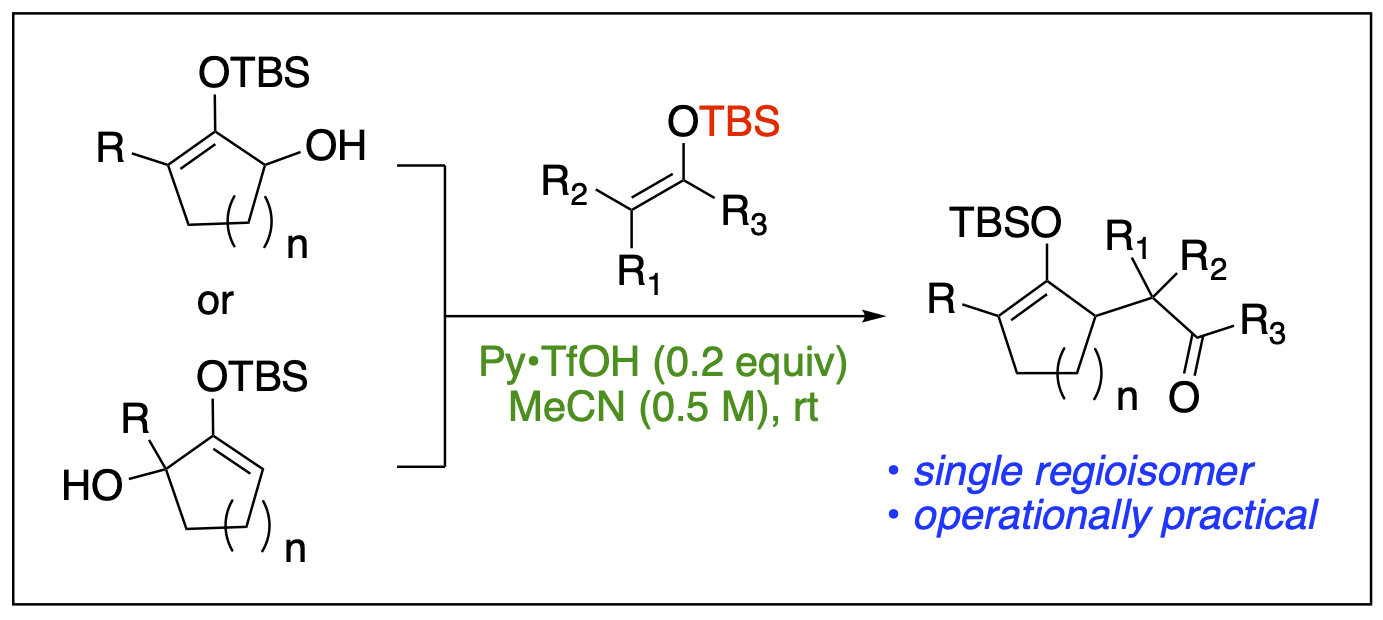
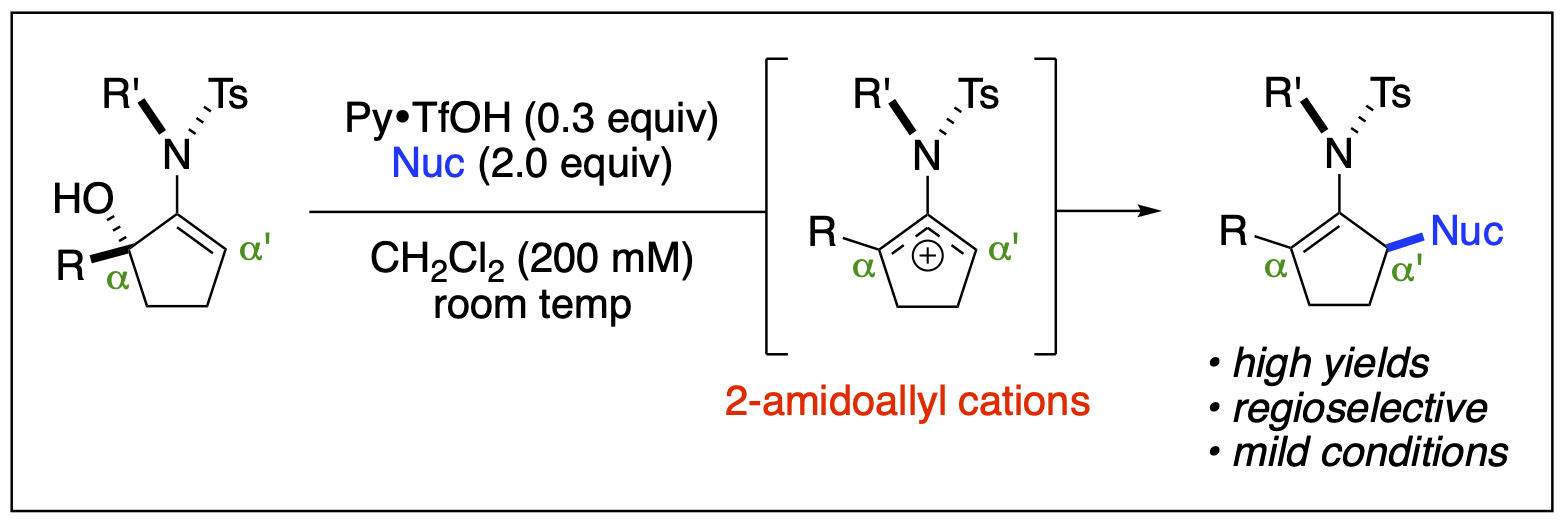
- Saputra, M. A.; Dange, N. S.; Cleveland, A. H.; Malone, J. A.; Fronczek, F. R.; Kartika, R. "Regioselective Functionalization of Enamides at the α-Carbon via Unsymmetrical 2-Amidoallyl Cations." Org. Lett. 2017, 19, 2414. DOI: 10.1021/acs.orglett.7b00962.
- Saputra, M. A.; Malone, J. A.; Cleveland, A. H.; Van Houten, J. P.; Kartika, R. "Bis(trichloromethyl)carbonate: Second Update." Encyclopedia of Reagents for Organic Synthesis 2017. DOI: 10.1002/047084289X.rb200.pub3.
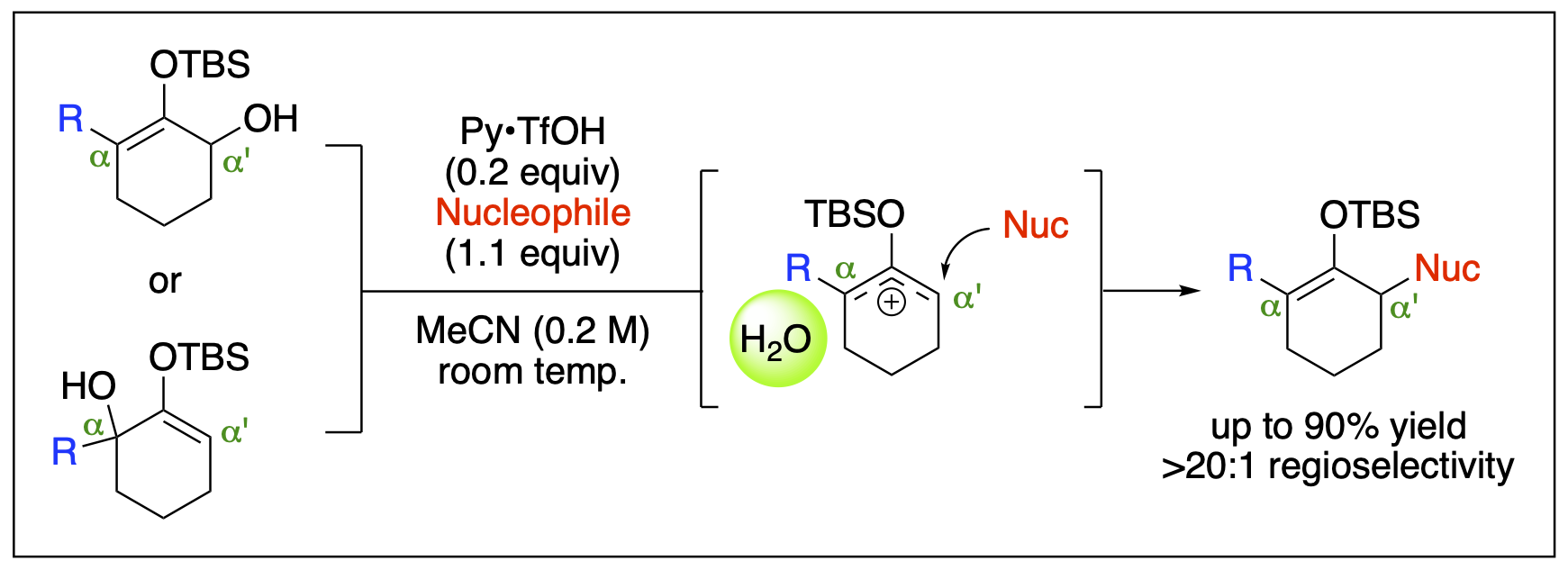
- Malone, J. A.; Cleveland, A. H.; Fronczek, F. R.; Kartika, R. "Effects of Solvent and Residual Water on Enhancing the Reactivity of 6-Membered Silyloxyallyl Cations towards Nucleophilic Addition." Org. Lett. 2016, 18, 4408. DOI: 10.1021/acs.orglett.6b02194.
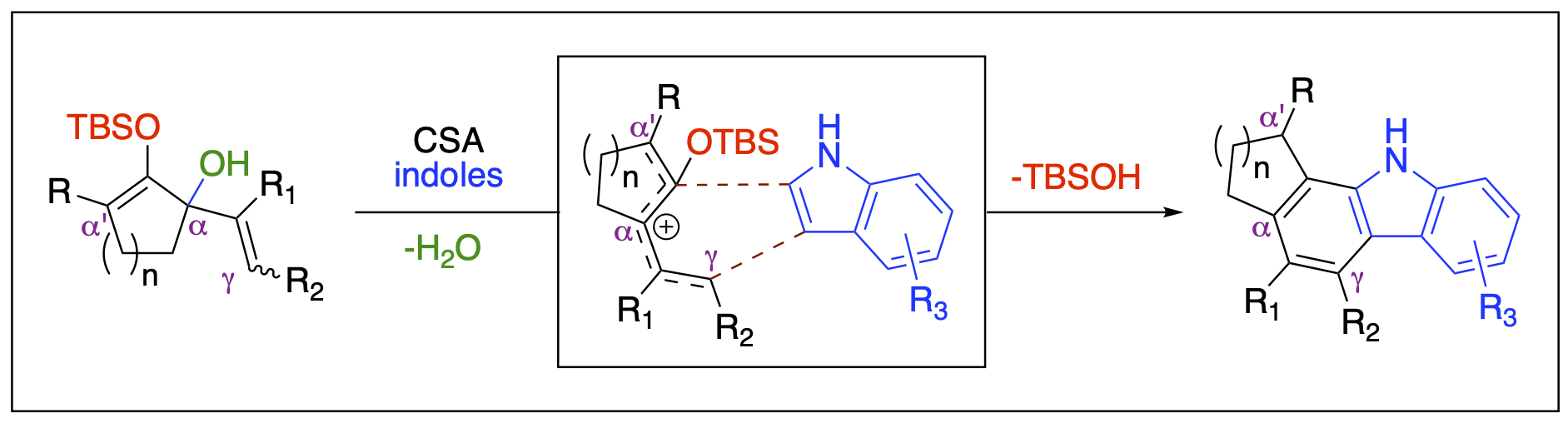
- Stepherson, J. R.; Ayala, C. E.; Tugwell, T. H.; Henry, J. L.; Fronczek, F. R.; Kartika, R. "A New Approach to Carbazole Annulation via Cascade Nucleophilic Addition – Cyclization Involving 2-Silyloxypentadienyl Cation." Org. Lett. 2016, 18, 3002. DOI: 10.1021/acs.orglett.6b01376.
- Ayala, C. E.; Dange, N. S.; Stepherson, J. R.; Henry, J. L.; Fronczek, F. R.; Kartika, R. "Functionalization of Silyldienol Ethers at the γ-Position via 2-Silyloxypentadienyl Cations." Org. Lett. 2016, 18, 1084. DOI: 10.1021/acs.orglett.6b00196.
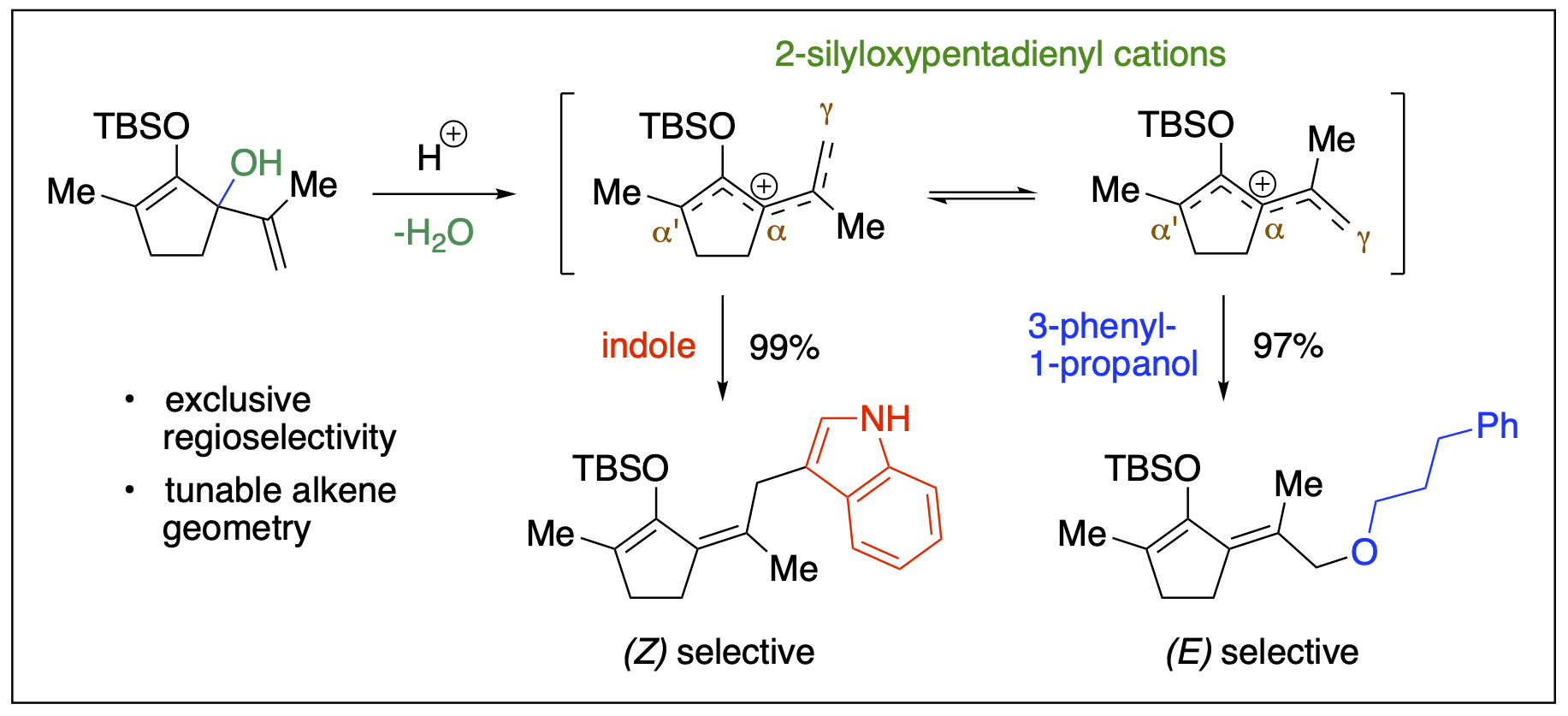
- Stepherson, J. R.; Fronczek, F. R.; Kartika, R. "An Expedient Synthesis of Functionalized 1,4-Diketone-Derived Compounds via Silyloxyallyl Cation Intermediates." Chem. Commun. 2016, 52, 2300. DOI: 10.1039/C5CC09763K.
- Stepherson, J. R.; Ayala, C. E.; Dange, N. S.; Kartika, R. "Nucleophilic Capture of Unsymmetrical Oxyallyl Cations with Indoles Under Mild Brønsted Acid Catalysis." Synlett 2016, 27, 320. DOI: 10.1055/s-0035-1560801.
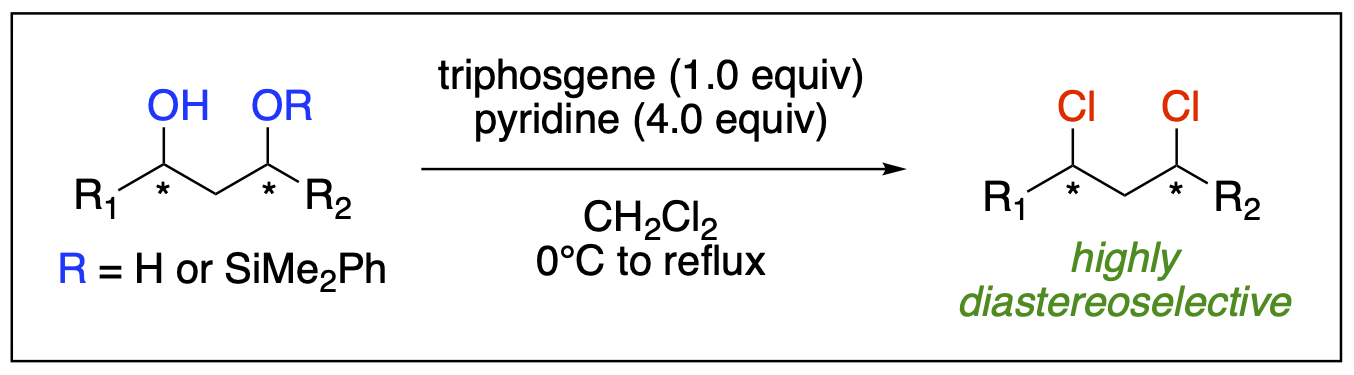
- Villalpando, A.; Saputra, M. A.; Tugwell, T. H.; Kartika, R. "Triphosgene-Pyridine Mediated Stereoselective Chlorination of Acyclic Aliphatic 1,3-Diols." Chem. Commun. 2015, 51, 15075. DOI: 10.1039/C5CC06365E.
- Saputra, M. A.; Ngo, Ly; Kartika, R. "Synthesis of Vinyl Chlorides via Triphosgene-Pyridine Activation of Ketones." J. Org. Chem. 2015, 80, 8815. DOI: 10.1021/acs.joc.5b01137.
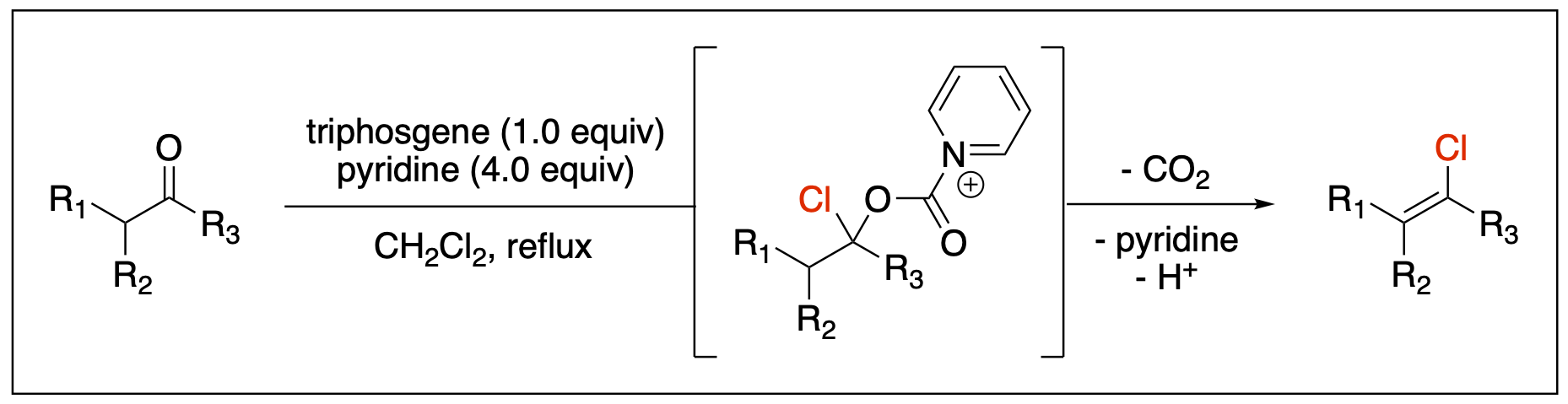
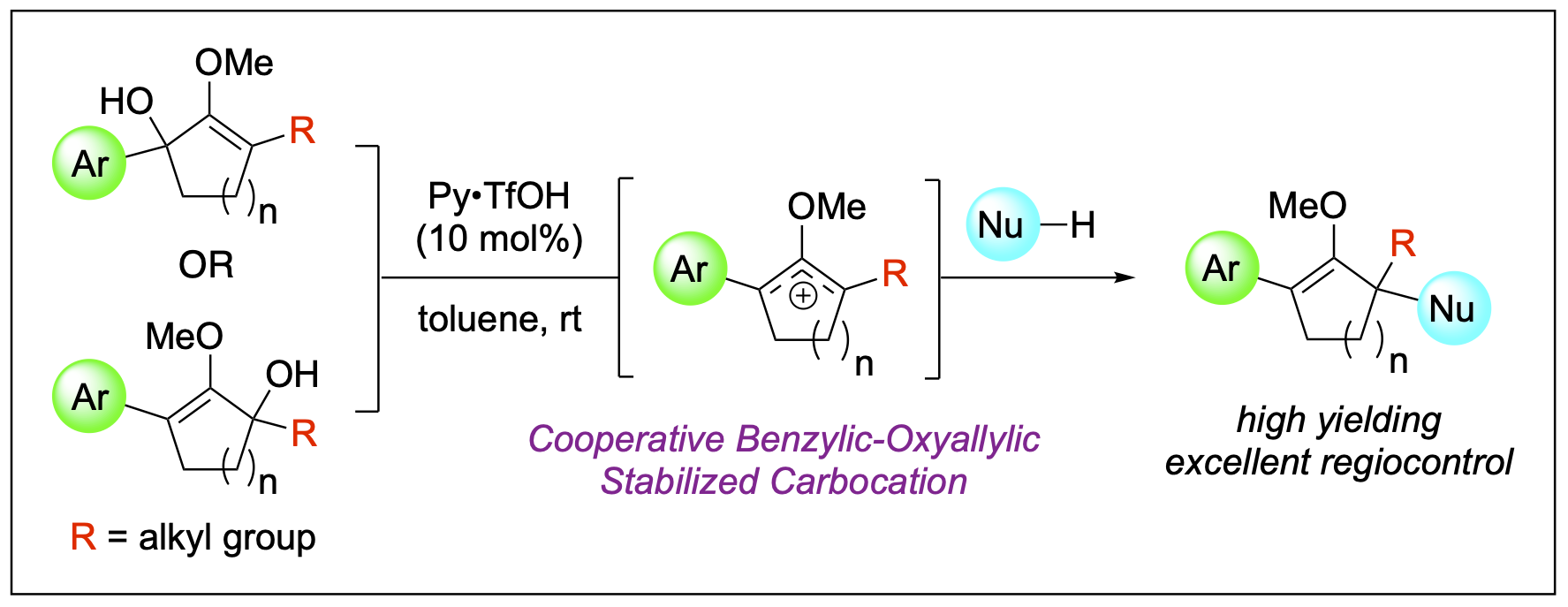
- Dange, N. S.; Stepherson, J. R.; Ayala, C. A.; Fronczek, F. R.; Kartika, R. "Cooperative Benzylic-Oxyallylic Stabilized Cations: Regioselective Construction of Alpha-Quaternary Centers in Ketone-Derived Compounds." Chem. Sci. 2015, 6, 6312. DOI: 10.1039/C5SC01914A.
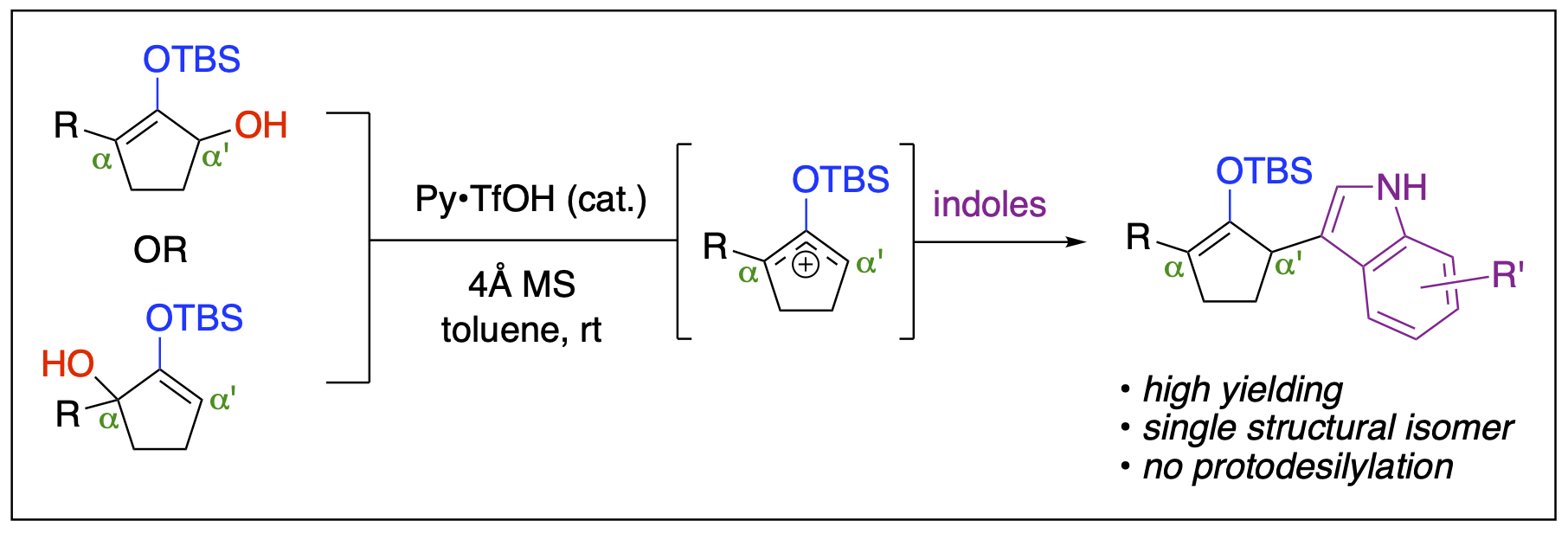
- Ayala, C. A.; Dange, N. S.; Fronczek; F. R.; Kartika, R. "Brønsted Acid Catalyzed α'-Functionalization of Silylenol Ethers with Indoles." Angew. Chem. Int. Ed. 2015, 54, 4641.
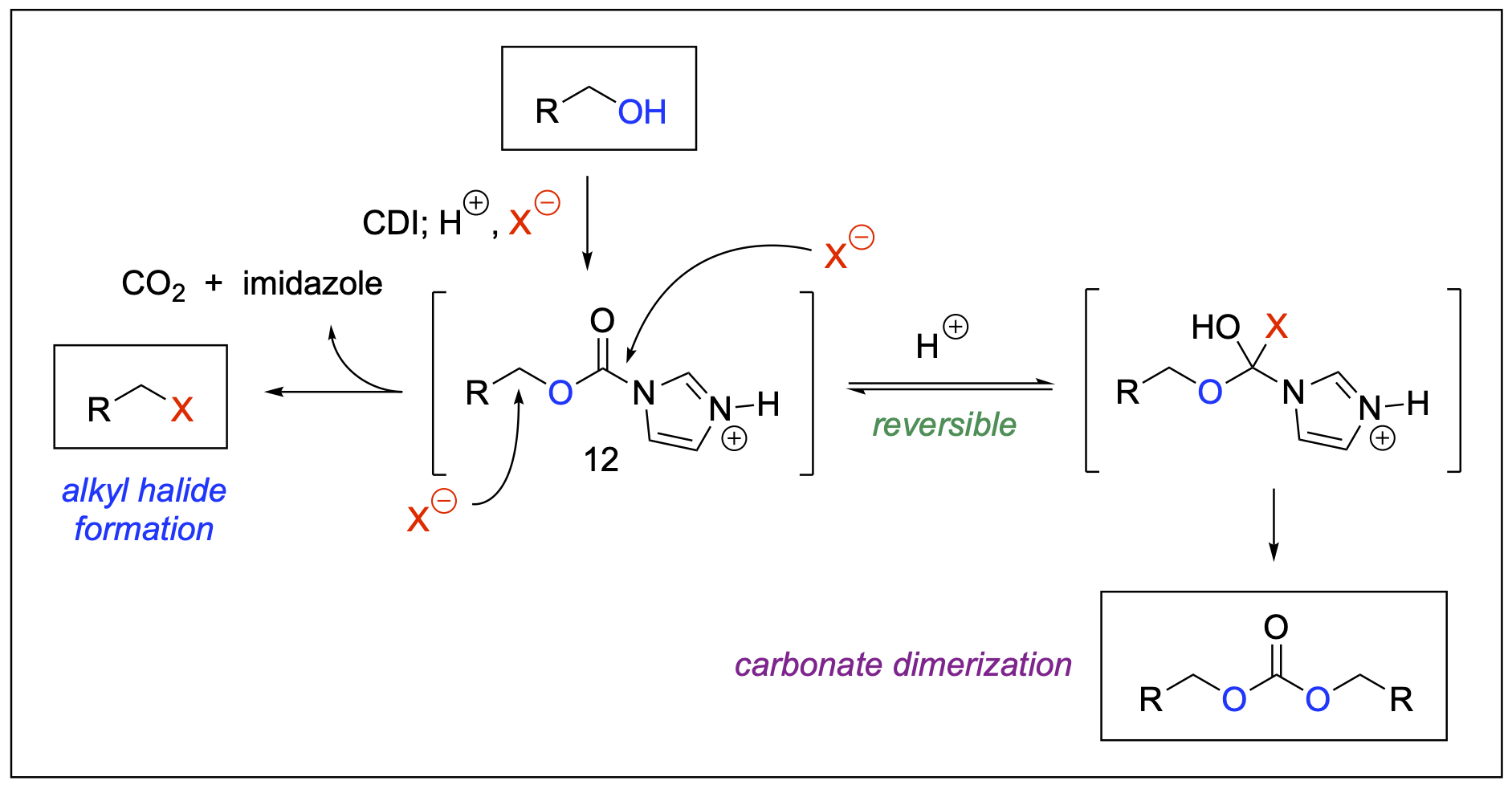
- Saputra, M. A.; Forgey, R. L.; Henry, J. L.; Kartika, R. "Mechanistic Insights Into Brønsted Acid-Induced Nucleophilic Substitution of Aliphatic Imidazole Carbamate with Halide Ions." Tetrahedron Lett. 2015, 56, 1392. DOI: 10.1016/j.tetlet.2015.01.098.
- Villalpando, A.; Ayala, C. E.; Watson, C. B.; Kartika, R. "Triphosgene-Amine Base Promoted Chlorination of Unactivated Aliphatic Alcohols." J. Org. Chem. 2013, 78, 3989. DOI: 10.1021/jo400341n. (Highlighted in Organic Chemistry Portal – ID: J42-Y2013-1010)
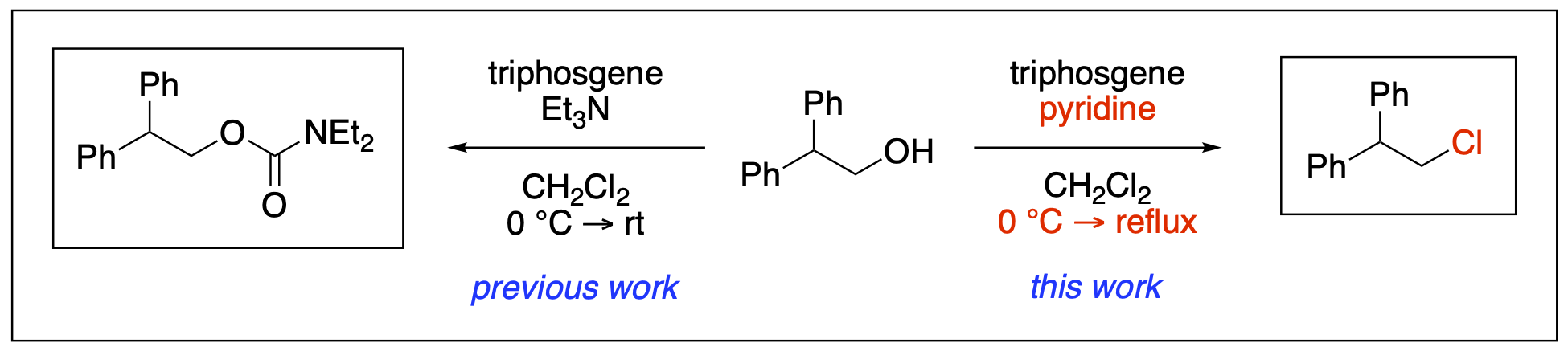
- Ayala, C. E.; Villalpando, A.; Nguyen, A. L.; McCandless, G. T.; Kartika, R. "Chlorination of Aliphatic Primary Alcohols via Triphosgene-Triethylamine Activation." Org. Lett. 2012, 14, 3676. DOI: 10.1021/ol301520d. (Highlighted in Organic Chemistry Portal – ID: J54-Y2012-2320)
Collaborative Research
With Prof. Gregg S. Pettis at LSU Biological Sciences
- Soares, N.; Huguet-Tapia, J.; Guan, D.; Clark, C.; Yang, K.-T.; Kluchka, O.; Thombal, R. S.; Kartika, R.; Badger, J.; Pettis. G. “Comparative Genomics of the Niche-Specific Plant Pathogen Streptomyces ipomoeae Reveal Novel Genome Content and Organization.” Appl. Environ. Microbiol. 2023, 89:e00308-23.
Before Louisiana State University
With Prof. David A. Spiegel at Yale University
- Vastl, J.; Kartika, R.; Park, K.; Cho, A. E.; Spiegel, D. A. "Peptidines: Glycine-Amidine-Based Oligomers for Solution- and Solid-Phase Synthesis." Chem. Sci. 2016, 7, 3317. DOI: 10.1039/C5SC03882K.
- Wang, T.; Kartika, R.; Spiegel, D. A. "Exploring Post-Translational Arginine Modification Using Chemically Synthesized Methylglyoxal Hydroimidazolones (MG-Hs)." J. Am. Chem. Soc. 2012, 134, 8958. DOI:10.1021/ja301994d.
With Prof. Richard E. Taylor at University of Notre Dame
- Kartika, R.; Gruffi, T. R.; Taylor, R. E. "Concise Enantioselective Total Synthesis of Neopeltolide Macrolactone Highlighted by Ether Transfer." Org. Lett. 2008, 10, 5047. DOI: 10.1021/ol802254z.
- Kartika, R.; Frein, J. D.; Taylor, R. E. "Electrophile-Induced Ether Transfer: Stereoselective Synthesis of 2,6-Disubstituted 3,4-Dihydropyrans." J. Org. Chem. 2008, 73, 5592. DOI: 10.1021/jo800704d.
- Kartika, R.; Taylor, R. E. "Electrophile-Induced Ether Transfer: An Expedient Route to 2-Cyano-Tetrahydropyrans." Heterocycles 2007, 74, 447. DOI: 10.3987/com-07-s(w)23.
- Kartika, R.; Taylor, R. E. "Electrophile-Induced Ether Transfer: Stereoselective Synthesis of 2,4,6-Trisubstituted Tetrahydropyrans." Angew. Chem. Int. Ed. 2007, 46, 6874.
- Kartika, R.; Taylor, R. E. "An Exhaustive Hydrogenation Strategy to Bicyclic Alkaloids." Chemtracts 2006, 19, 385.
- Liu, K.; Taylor, R. E.; Kartika, R. "Electrophile-Induced Ether Transfer: A New Approach to Polyketide Structural Units." Org. Lett. 2006, 8, 5393. DOI: 10.1021/ol0623318.
With Prof. Douglas A. Klumpp at California State Polytechnic University Pomona
- Klumpp, D. A.; Zhang, Y.; Do, D.; Kartika, R. "Reactions of Acenaphthenequinone and Aceanthrenequinone with Arenes in Superacid." Applied Catalysis, A: General 2008, 336, 128. DOI: 10.1016/j.apcata.2007.08.036.
- Zhang, Y.; Briski, J.; Zhang, Y.; Rendy, R.; Klumpp, D. A. "Superacid-Catalyzed Reactions of Olefinic Pyrazines: an Example of Anti-Markovnikov Addition Involving Superelectrophiles." Org. Lett. 2005, 7, 2505. DOI:10.1021/ol050900q.
- Klumpp, D. A.; Rendy, R.; Zhang, Y.; McElrea, A.; Gomez, A.; Dang, H. "Reactive Dications: The Superacid-Catalyzed Reactions of Alkynes Bearing Adjacent N-Heterocycles or Amine Groups." J. Org. Chem. 2004, 69, 8108. DOI: 10.1021/jo040218u.
- Klumpp, D. A.; Rendy, R.; McElrea, A. "Superacid Catalyzed Ring-Opening Reactions Involving 2-Oxazolines and the Role of Superelectrophilic Intermediates." Tetrahedron Lett. 2004, 45, 7959. DOI: 10.1016/j.tetlet.2004.08.109.
- Klumpp, D. A.; Rendy, R.; Zhang, Y.; Gomez, A.; McElrea, A. "Dicationic Intermediates Involving Protonated Amides: Dual Modes of Reactivity Including the Acylation of Arenes." Org. Lett. 2004, 6, 1789. DOI: 10.1021/ol049512z.
- Rendy, R.; Zhang, Y.; McElrea, A.; Gomez, A.; Klumpp, D. A. "Superacid-Catalyzed Reactions of Cinnamic Acids and the Role of Superelectrophiles." J. Org. Chem. 2004, 69, 2340. DOI: 10.1021/jo030327t.
- Zhang, Y.; McElrea, A.; Sanchez, G. V., Jr.; Do, D.; Gomez, A.: Aguirre, S. L.; Rendy, R.; Klumpp, D. A. "Dicationic Electrophiles from Olefinic Amines in Superacid." J. Org. Chem. 2003, 68, 5119. DOI: 10.1021/jo030024z.