Our Research
Our research involves a problem solving approach to answering fundamental questions in chemistry, as well as development and application of novel strategies (chemical, instrumental, and mathematical) for measurement science. The overall goal of our research is to provide improved strategies for answers to important questions of chemical origin using the problem solving approach common to analytical measurements. Although the primary focus of our research is in the broad area of chemistry, most of our studies emphasize the more specific areas of analytical, materials, and environmental sciences, with a strong emphasis on bioanalytical chemistry. Some examples of current research projects are provided below.
- development of ionic liquids (ILs) and GUMBOS (group of uniform materials based on organic salts) for applications in materials, biomedical, and bioanalytical chemistry;
- synthesis and production of nanoparticles (particularly nanoGUMBOS) for novel biomedical applications and bioanalytical measurements;
- development of novel analytical schemes for protein analyses, particularly those relevant to MALDI and SDS-PAGE analyses;
- development of sensors and sensor arrays using ionic liquids and GUMBOS for rapid bioanalytical measurements;
- studies of the influence and applications of guest/host chemistry, e.g., organized media, to chemical systems of analytical interests;
- development and application of molecular spectroscopy, particularly absorption and fluorescence, for bioanalytical measurements.
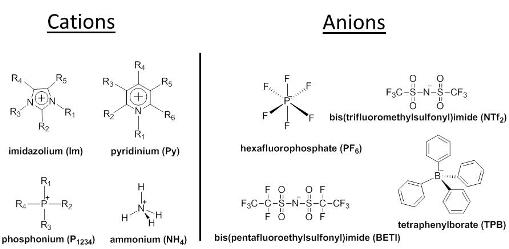
The definition of ionic liquids as organic salts that melt below 100°C is arbitrary and more reflective of the desire of researchers to use these salts as solvents at or near room temperature. To achieve this goal, many ionic liquids use an organic ion and an inorganic counterion to provide incompatibility in the size of the counterions, which is suggested to lead to asymmetric packing and thus to liquids rather than solids as normally observed for salts. The figure below provides examples of cations and anions which can be used for preparing ionic liquids. It is estimated that ~1018 ternary ionic liquids, many of which are solids at room temperature, can be synthesized. This is a large number which allows the preparation and use of an enormous number and variety of ILs and GUMBOS, and thus also nanoGUMBOS.
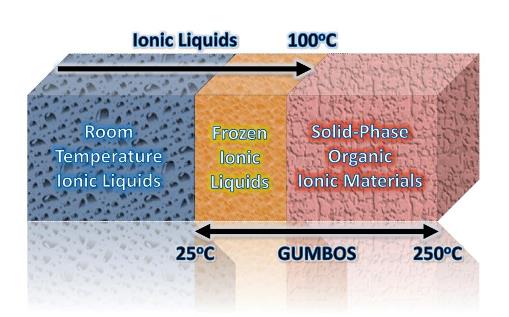
In contrast to typical ionic liquid research, our research involves the use of the tunable properties of low melting organic salts (GUMBOS) to develop novel solid phase materials and ultimately for the development of nanoGUMBOS for bioanalytical applications. We note that the acronym, GUMBOS, is both singular and plural, and encompasses frozen ionic liquids, i.e. organic salts with melting points between 25°C and 100°C. However, in our research we use the more general term, GUMBOS, to expand the possible applications by encompassing solid phase organic salts with melting points up to 250 °C as depicted in the figure below which contrasts the melting point of ILs and GUMBOS. Thus, we are developing solid phase materials with the same tunability as ionic liquids, but with a wider range of melting points for solid phase applications.
Since we are primarily interested in solids rather than liquids in our GUMBOS research, there is no logical reason why we cannot extend our melting point range to 250°C, while maintaining the tunability which is inherent to ionic liquids. In addition, we can employ other organic and inorganic materials as counterions. We note that it is this tunability that allows GUMBOS to be imparted with multiple properties such as fluorescence, magnetism, biocompatibility, and biodegradability. For example, variations in the anion can produce compounds which are extremely hydrophobic or alternatively, extremely hydrophilic, or even somewhere in between. Examples of the latter are ionic liquids with very narrow solubility ranges, e.g. an ionic liquid which is insoluble in water, soluble in methanol, and insoluble in hexane. The significant point to be made here is that such properties clearly defy chemical intuition. It is also significant that these properties can be uniformly imparted into GUMBOS, as well as a single nanoparticle, i.e. nanoGUMBOS.
From the above discussion, it should be clear that if we desire fluorescent GUMBOS, we can prepare our GUMBOS using a fluorescent cation. Similarly, if we desire GUMBOS that are both fluorescent and magnetic, we can use a fluorescent cation and a paramagnetic anion. For example, we have recently demonstrated how three parameters, i.e. cancer targeting, luminescence, and magnetism, can be uniformly incorporated into a single nanoparticle. Thus, the assembly of nanoparticles from GUMBOS represents a truly general approach for construction of nanoparticles of varying properties. While the focus of our research is on development of these materials for bioanalytical applications such as imaging and drug delivery, it should be clear that other properties can be incorporated. Finally, we note that it is the combination of simplicity and tunability which dictate the significance of this approach.
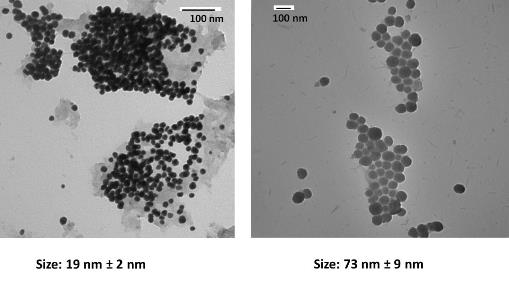
We are exploring a number of different approaches to developing nanoparticles from GUMBOS. The figure below shows the TEM micrographs of the nanoparticles obtained when using 2HP-?-CD as a template with ultrasound at two different degrees of molecular substitutions for 2HP-?-CD (MS=0.6 and MS=0.8). The spherical nanoparticles with an average size of 19 nm and a standard deviation of 2 nm as pictured in the TEM micrographs (a) were obtained when using 2HP-?-CD (MS=0.6) at a concentration of 4 mM of reactants. However, the size increases to an average of 73 nm and a standard deviation of 9 nm for 2HP-?-CD with molecular substitution of 0.8. A similar preparation was done using 1 mL and 100 ?L of tert-butanol to investigate the possible effects of alcohol on the size of the synthesized nanoGUMBOS and subsequently on the role of 2-hydroxypropyl-?-cyclodextrin to form a complex with the nanoGUMBOS. The total volume of sample was held constant at 10 mL and MS=0.6 2HP-?-CD tested. Considerably smaller particles were obtained using this approach. In addition to examining a variety of substituted cyclodextrins, we arel also examining nanoparticles produced with these cyclodextrins in the presence of alcohols of various structures. Preliminary data shows excellent control using this approach. This approach follows from chemistry explored in our laboratory many years ago. Other cyclodextrins will also be tested.
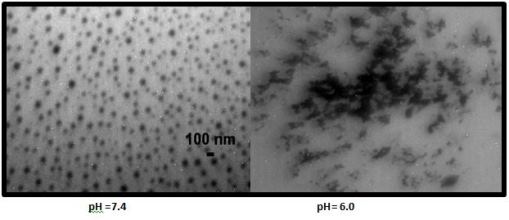
We have also synthesized numerous nanoGUMBOS which are stimuli responsive. As one example, consider the synthesis of GUMBOS using two pH sensitive anions [sodium deoxycholate (NaDC) and sodium chenodeoxycholate (NaChDC)] in combination with phthalocyanine as the cation. In both cases, we were able to produce GUMBOS and nanoGUMBOS which were sensitive to variations in pH values, as well as sensitivity to a magnetic field. As an example, consider the results shown in the figure below from a study of variations in the pH environment using the phthalocyanine derivative of ChDC- (PTChDC). At a pH value of 7.4, this compound formed nice spherical nanoGUMBOS of ~100 nm. At pH 6.0, these nanoGUMBOS disintegrated into an amorphous solid material. Clearly, this compound is greatly stimulated at acidic pH values. It should be clear that this property would be very useful as a drug delivery system for cancerous or infectious tissues since these tissues tend to be more acidic than healthy tissues. It should also be clear that it is possible to tune the pH value at which the nanoGUMBOS disaggregate by using anions of different pKa values. Such experiments represent the next phase of our studies in this area.
The above cited studies are only representative examples of our ongoing research focusing primarily of ILs and GUMBOS chemistry. A number of other projects are ongoing in the general areas of bioanalytical, materials, and biomedical chemistry. Please consult any number of references cited on our webpage to obtain an overview of our complete body of work. We particularly direct you to the reference cited below [1] for an overview of our GUMBOS research.
- Warner, I.M., B. El-Zahab, and N. Siraj, Perspectives on Moving Ionic Liquid Chemistry into the Solid Phase. Analytical Chemistry, 2014.