Research
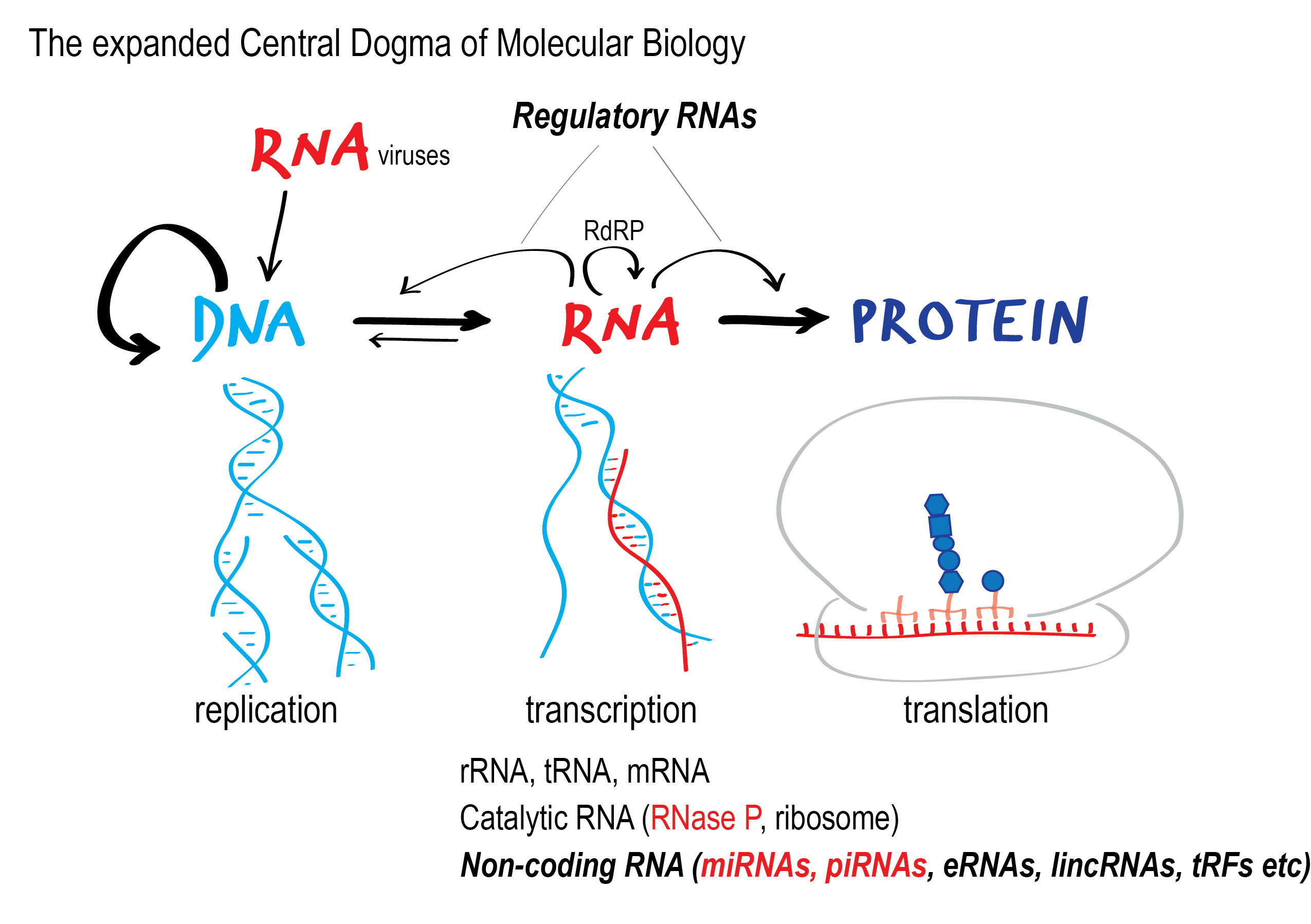
Gene expression at the post-transcriptional level is controlled by ribonucleoprotein complexes
The cell controls gene expression by when and how much a gene is transcribed (transcriptional control), and also by regulating if and how the message RNA is spliced, processed, edited or chemically modified, stabilized, transported and eventually translated in the ribosomes (post-transcriptional control).
As soon as a gene is transcribed, the mRNA is bound by proteins that control its fate, and thus post-transcriptional control is all about the formation and the function of ribonucleoprotein complexes, or RNPs. Importantly, beyond being a carrier of information, non-protein-coding RNA is often a functional partner in these RNPs. New non-coding RNAs (ncRNAs), small and long, are constantly being discovered through large scale projects (Human Genome Sequencing, ENCODE) and targeted research, and “old” ncRNAs are given new functional attributes.
CLIP-Seq illuminates the function of regulatory RNPs at the molecular level – Previous work on Piwi proteins and piRNAs
A major challenge in the study of RNPs and therefore in defining the regulatory functions that RNPs carry out, is identifying the molecular details of RNA binding by RBPs in vivo. The exact sequence that is bound by the RBP is only one aspect of that: very often the binding results in the formation, or the modulation of an RNA secondary structure. High-throughput sequencing after Crosslinking and Immunoprecipitation (CLIP-Seq) uncovers both the bound sequence at a nucleotide resolution, and also provide evidence for the involvement of RNA secondary structure in the function of the RNP. The chimeric CLIP-Seq variant (cCLIP-Seq) captures the transient intra- and/or inter- molecular duplexes that are formed and/or modulated at the protein contact site (Vourekas and Mourelatos, Methods in Molecular Biology 2013; Alexiou et al. Methods in Molecular Biology 2018).
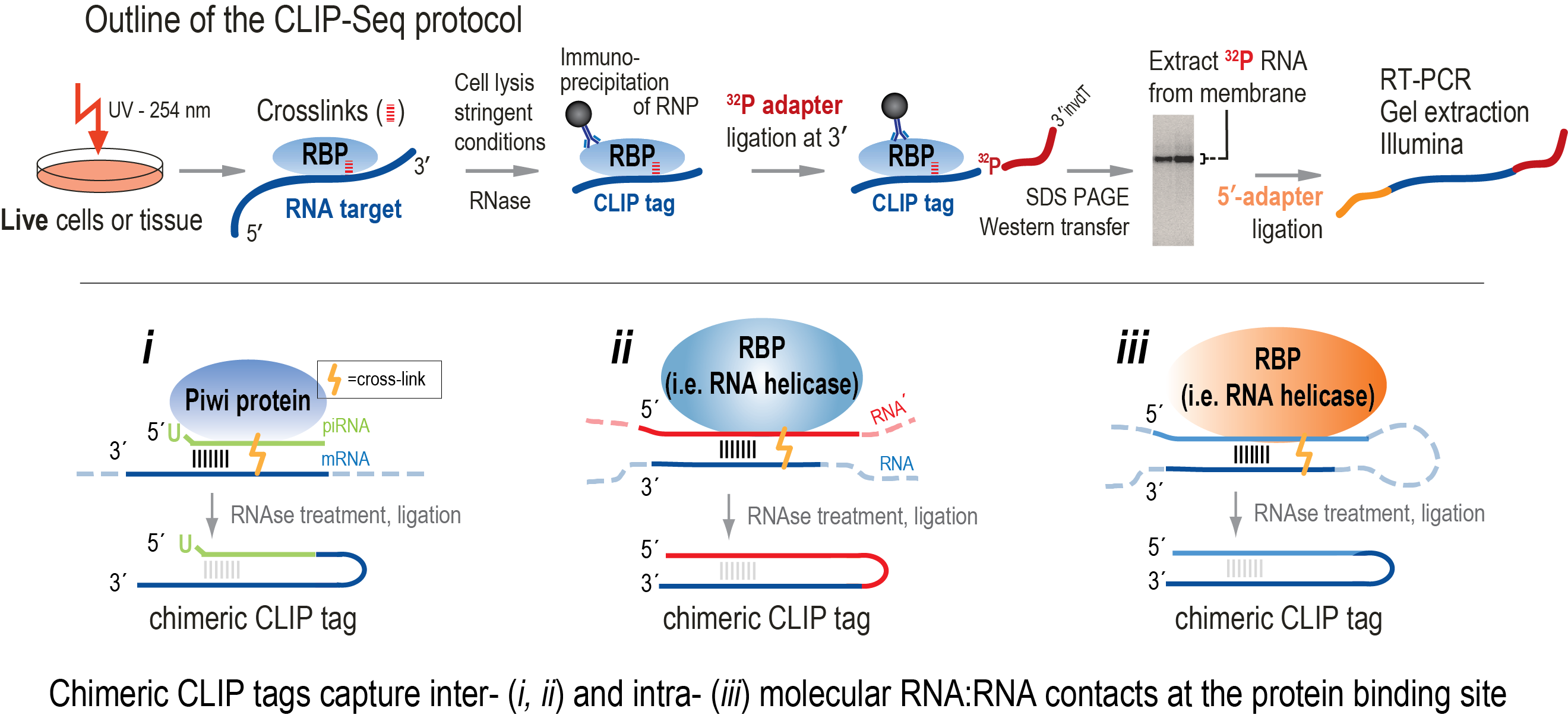
Using CLIP-Seq, we previously uncovered transient piRNA precursor intermediates bound on Piwi proteins, which allowed us to elucidate the process that produces Piwi
bound mature piRNAs (Vourekas et al. NSMB 2012). Furthermore, CLIP-Seq for a helicase involved in piRNA biogenesis allowed us to
hypothesize that piRNA biogenesis proceeds through consecutive cleavage events of the long piRNA precursor transcript (Vourekas et al. Genes & Development 2015), a process later named “phasing”.
By analyzing Piwi protein chimeric reads, we revealed a mechanism that segregates
mRNAs in germline RNP granules, mediated by piRNA complementarity. We showed that
Piwi proteins loaded with piRNAs of diverse sequences are localized in germ granules,
and piRNAs form a seed sequence dependent non-discriminatory “adhesive trap” that captures
mRNAs by forming numerous and random low complementarity piRNA:mRNA contacts (Vourekas et al . Nature 2016). Neither the small RNAs or the base-paired mRNA sequences are conserved, thus this
mechanism is not sequence specific. Strikingly, we found that although base-paired
mRNA sequences appear randomly and with the same frequency in all mRNAs, germ granule
mRNAs are longer and more abundant, and therefore they form more contacts with piRNAs,
and so their entrapment in the granules is enhanced. We call this phenomenon piRNA adhesion, to separate it from purposeful (i.e. through conserved RNA sequences) small RNA
directed complementary sequence targeting (Vourekas et al . Nature 2016). We are currently investigating how conserved this mechanism is, by studying the
mouse germ granules and Miwi/piRNA RNP complexes in the chromatoid body.
The RNA adhesion hypothesis for RNP formation and function – The role of RNA helicases
After describing the piRNA adhesion mechanism, it became apparent that this mechanism likely exists in a more general form: RNA:RNA contacts between any RNAs that are in proximity within RNP granules; this hypothesis can be called RNA adhesion. Small or long, non-coding or protein coding RNAs may be recruited to RNP complexes as “RNA adhesive traps” that operate via low complementarity RNA:RNA contacts. Importantly, work from the Roy Parker lab recently showed that RNA alone in high enough concentration can phase separate on its own, and that stress granules indeed contain mRNAs that are on average longer than the mRNAs that were depleted from the stress granules and remained in the cytoplasm, which is in full agreement with the hypothesis that we formed based on our 2016 findings.
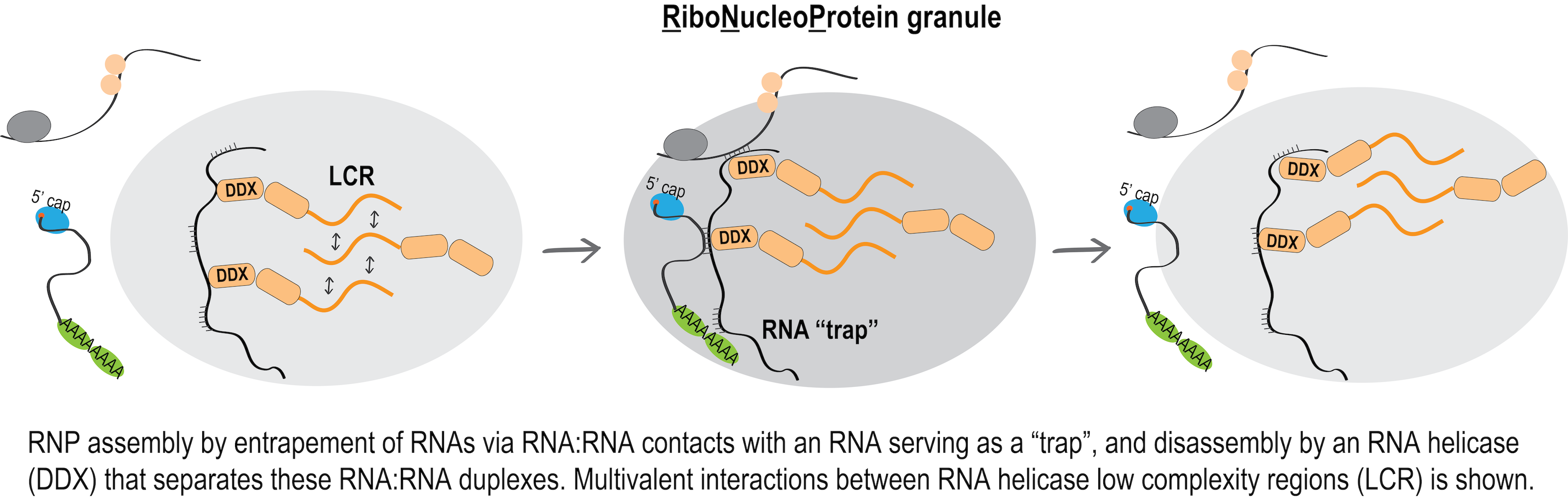
We are currently exploring the hypothesis that RNA helicases, as core components of
RNP granules, play a key role in the remodeling of these transient RNA duplexes, i.e.
by promoting their formation to facilitate RNP assembly, or by dissociating RNA duplexes
during RNP disassembly. Despite the wealth of information from genetics and in vitro
assays, we still lack critical information on how individual RNA helicases identify
their RNA targets, and what they actually do to these RNAs in vivo. To address these
points, we have initiated a study of a core stress granule factor, DEAD-box RNA helicase
DDX3X. Our vision is to expand the same approach for critical RNP granule RBPs for
which we know little or nothing about the in vivo bound RNA targets.
We are currently studying the role of DDX3X in the context of stress (e.g. nutrient
deprivation, ER stress), and we are finding that in association with the eIF3 complex,
DDX3X is involved in stress related alternative translation initiation events (in
preparation). In a separate project, DDX3X is involved in translation initiation of
HIV mRNAs during reactivation from latency (in preparation).
Stress related small RNAs and beyond
Our studies have lead us to look closer in RNA regulatory events during stress. The first-tier response to stress is at the level of inhibition of translation initiation, via phosphorylation of eIF2α, which results in the reduced availability of the ternary complex. The cell is regulating gene expression primarily at the level of translation, as a fast response essential for preserving precious resources in stress conditions. At the same time, stress related small RNAs such as tRNA fragments represent a novel class of small regulatory RNAs whose biogenesis and protein effectors are still largely elusive. We are strongly intrigued by these small RNAs, and we are currently evaluating various experimental approaches to uncover their biogenesis and functional complexes.