Research
1. Regulatory mechanisms of cytoskeletal reorganization during epithelial tube formation
One mechanism by which tubular organs form is through the budding of cells from a polarized epithelial sheet: a subset of cells bud out of the plane of the epithelium to become the end of a new tube (Fig. 1). This process, known as tissue invagination by budding, is observed in a simple epithelial tube such as Drosophila embryonic salivary gland (SG), as well as during the branching morphogenesis of more complex organs, including the mammalian lungs and kidney. Correct tissue invagination is critical for forming well-developed tubular organs, as defects in this initial step of tube formation could affect the shape or positin of the entire tube.
A crucial cell shape change during tissue invagination is apical constriction, a widespread cellular process where the apical side of the cell becomes smaller than the other sides (Fig. 2). During tissue invagination, apical constriction creates cells with a wedge-like shape that can fit together to form the curved wall of the tube, similar to shaped bricks in an archway. Defective apical constriction causes failure or disruption of formation of many tubular organs.
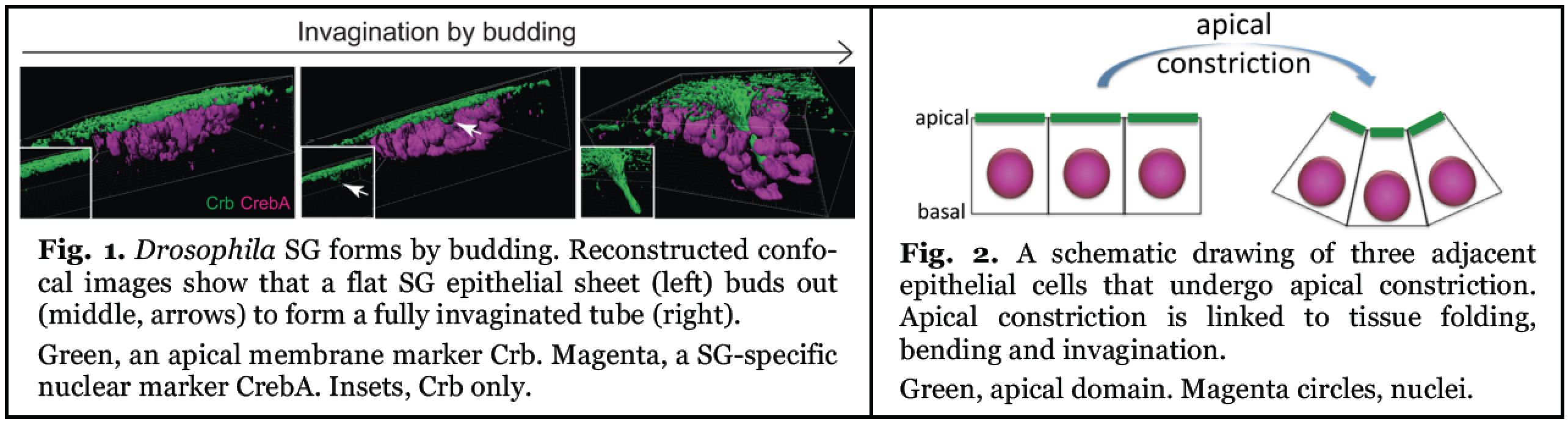
Fig. 1. Drosphilia SG forms by budding. Reconstructed confocal images show that a flat SG epithelial sheet (left) buds out (middle, arrows) to form a fully invaginated tube (right). Green, an apical membrane marker Crb. Magenta, a SG-specific nuclear marker CrebA. Insets, Crb only.
Fig. 2. A schematic drawing of three adjacent epithelial cells that undergo apical constriction. Apical constriction is linked to tissure folding, bending and invagination. Green, apical domain. Magenta circles, nuclei.
The critical role of apical constriction in tube formation has been recognized for decades in both invertebrates and vertebrates. However, we only recently began to understand the molecular mechanisms of apical constriction, in large part from studies using Drosophila. This has revealed a novel and unexpected role for a distinct myosin structure created in response to cell-cell signaling and regulated by apical Rho-associated kinase (Rok). This myosin structure, which forms in the center of the apical end of cells (apicomedial myosin), is highly pulsatile and generates the pulling force to drive apical constriction (Fig. 3).
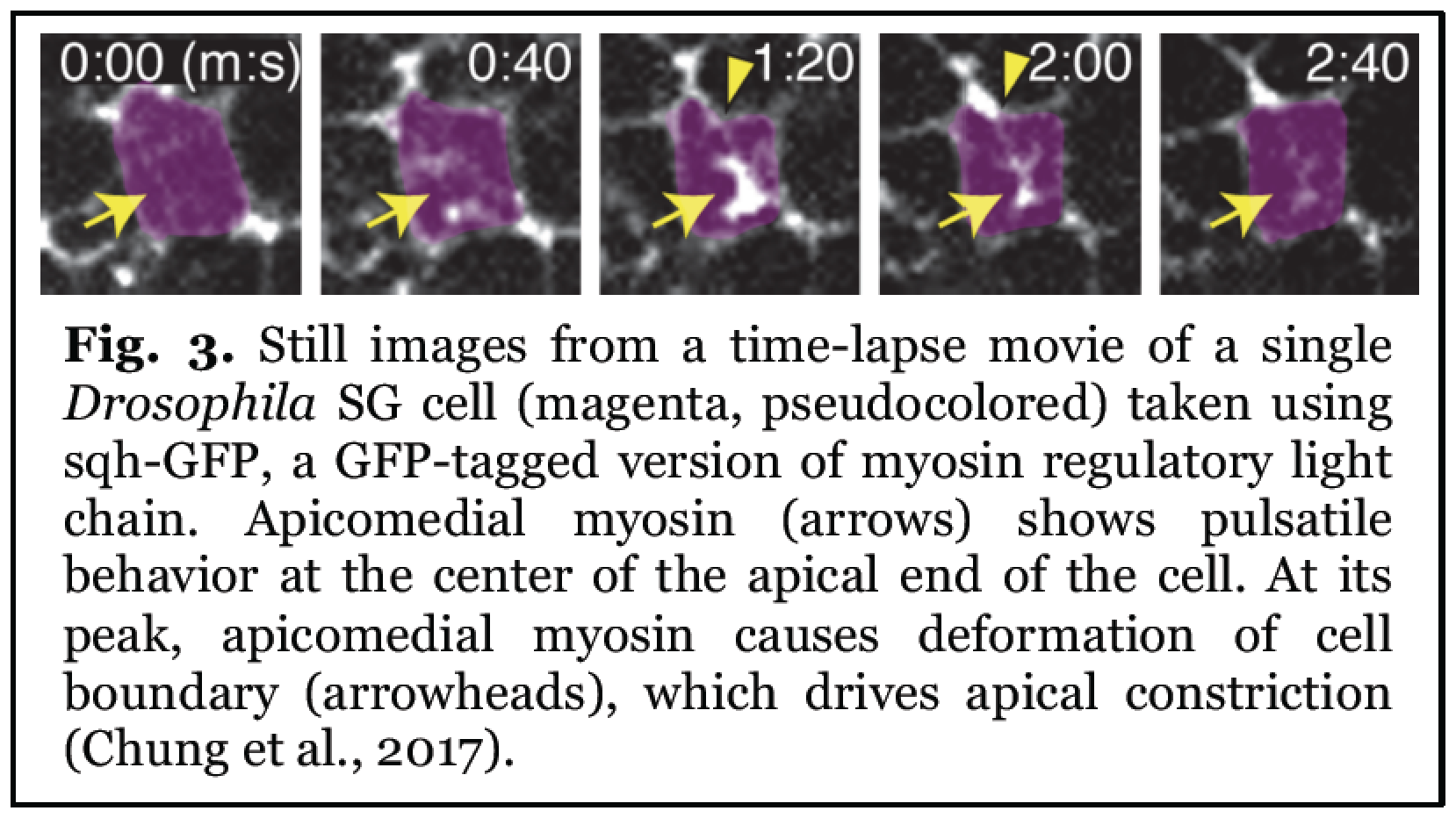
Still images from a time-lapse movie of a single Drosophila SG cell (magenta, pseudocolored) taken using sqh-GFP, a GFP-tagged version of myosin regulatory light chain. Apicomedial myosin (arrows) shows pulsatile peak, apicomedial myosin causes deformation of cell boundary (arrowheads), which drives apical constriction.
We use the Drosophila embryonic SG as a model system to reveal the regulatory mechanisms of apical constriction during tissue invagination (Fig. 4). Our previous studies discovered a pathway that drives apical constriction during SG invagination: the SG transcription factor Fork head regulates expression of the secreted ligand Folded gastrulation (Fog), which promotes accumulation of Rok and apicomedial myosin formation (Fig. 4; Chung et al., 2017). We further showed that the G protein-coupled receptor (GPCR) Smog transduces Fog signal to regulate this process (Vishwakarma et al., 2022) and also revealed a key role of microtubule- and Rab11-dependent intracellular trafficking in regulating apical constriction (Le and Chung, 2021). We are currently elucidating how GPCRs, RhoGEFs, and RhoA function to precisely control epithelial tube formation.
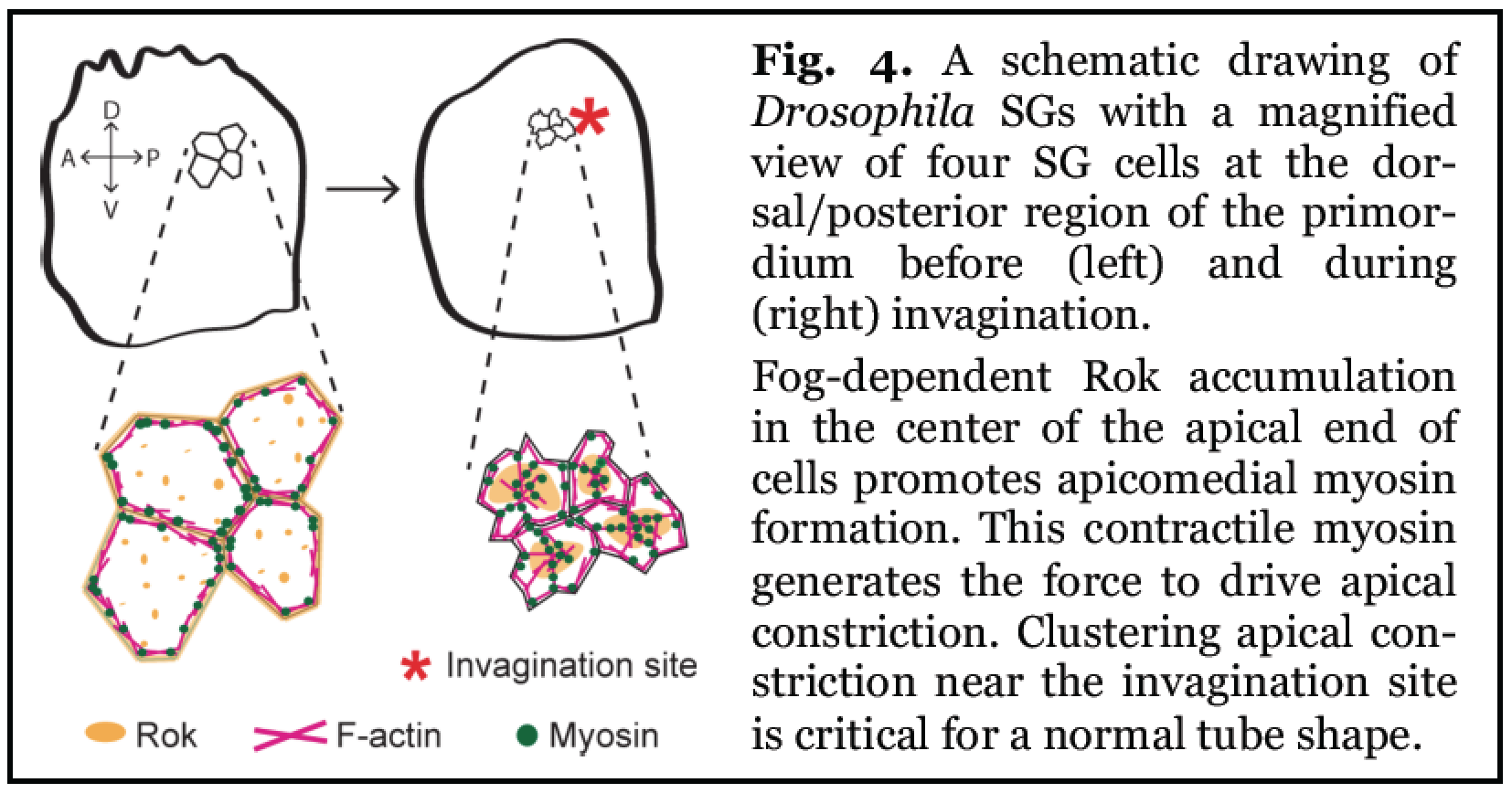
Fig. 4. A schematic drawing of Drosophila SGs with a magnified view of four SG cells at the dorsal/posterior region of the primordium before (left) and during (right) invagination.
Fog-dependent Rok accumulation in the center of the apical end of cells promotes apicomedial myosin formation. This contractile myosin generates the force to drive apical constriction. CLustering apical constriction near the invagination site is crirical for a normal tube shape.
2. Coordination of cell cycle and morphogenesis during organ formation
Organ formation requires precise regulation of cell cycle and morphogenetic events. Using the Drosophila embryonic SG as a model, we uncovered a key role for the SP1/KLF transcription factor Huckebein (Hkb) in linking cell cycle control with tissue morphogenesis (Matthew et al., 2024). TIn hkb mutants, SGs show defects in invagination positioning and organ size, caused by abnormal cell death. Normally, SG development progresses through endoreplication (endocycle) from distal to proximal regions. In contrast, hkb mutant cells undergo abnormal cell division, triggering cell death and disrupting organ formation. Mechanistically, Hkb suppresses the expression of key cell cycle regulators and pro-apoptotic genes in the SG. Remarkably, knockdown of cyclin E or cyclin-dependent kinase 1, or overexpression of fizzy-related, can rescue most of the morphogenetic defects observed in hkb mutants. These findings reveal that Hkb is a critical transcriptional regulator that ensures proper organ formation by promoting endoreplication and preventing aberrant cell division and death (Fig. 5). We are now expanding this work to investigate how Hkb functions in other tissues, aiming to uncover broader principles of cell cycle regulation in organ development.

3. Organization of the apical extracellular matrix (aECM) during organ formaion
The ECM consists of an organized meshwork of proteoglycans and fibrous proteins. During tubular organ formation, aECM components are produced by the surrounding epithelium and secreted apically. Compared to the well-established roles of the basal ECM in tissue morphogenesis and homeostasis, the aECM has been considered as a passive protective barrier against pathogens and environmental toxins. Increasing evidence, primarily from studies in C. elegans and Drosophila, suggests that the aECM also actively contributes to epithelial morphogenesis. In our recent work, we demonstrated a requirement for sulfation in organizing aECM during tubulogenesis in Drosophila. We identified and characterized the organization of some of the first known components of the non-chitinous aECM in the Drosophila salivary gland tube, namely the zona pellucida (ZP) domain proteins Piopio and Dumpy (Fig. 6; Woodward, Matthew et al., 2025). We are currently testing the conserved roles of sulfation and key aECM proteins in aECM organization in other Drosophila organs and human SG organoids.
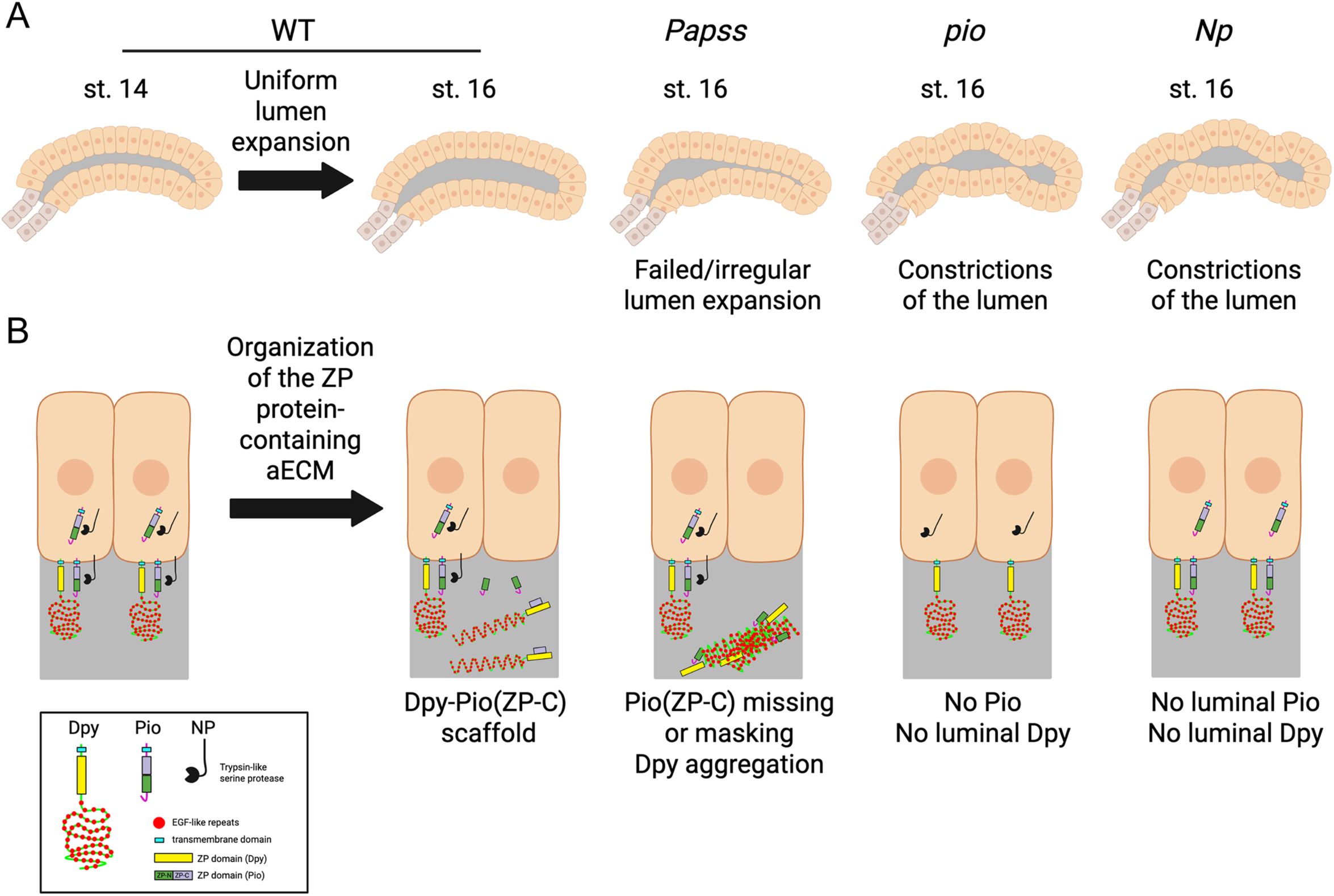
Fig. 6. A model for the role of Papss, a key sulfation enzyme, in the organization of the aECM in the Drosophila SG.
(A) Cartoon diagram showing normal lumen expansion in WT SG and defective luminal morphology in Papss, pio, and Np mutants.
(B) Organization of the ZP domain protein-containing aECM in the SG. In WT, the ZP-C fragment of Pio (which is cleaved by Np and furin proteases) forms a complex with Dpy to build a filamentous scaffold within the lumen. In Papss mutants, this fragment is absent from the lumen at stage 16, and the Dpy-containing aECM structure becomes aggregated and highly condensed. The absence of luminal Pio in pio and Np mutants results in the loss of luminal Dpy and lumen constrictions.