Ongoing Projects
We are currently developing new studies that use smartwatches and mobile health apps to support the transition from self-regulation to long-term habit formation. Stay tuned!

Completed Projects
Physical Activity and Percussive Massage Therapy for Reducing Pain in Older Women (MAPAP) |
||
|
Funder: Therabody Inc. ClinicalTrials.gov ID: NCT07056335 Principal Investigator: Shiyu Li, PhD; David E. Conroy, PhD (Faculty Advisor) Participants: women aged 65+ Setting: digital intervention Sample Size: 108 Research Question: Should a daily self-monitoring intervention focus on physical activity, percussive massage therapy, or both to promote engagement in those behaviors and reduce pain intensity and interference in older women. |
|
|
|
Description:
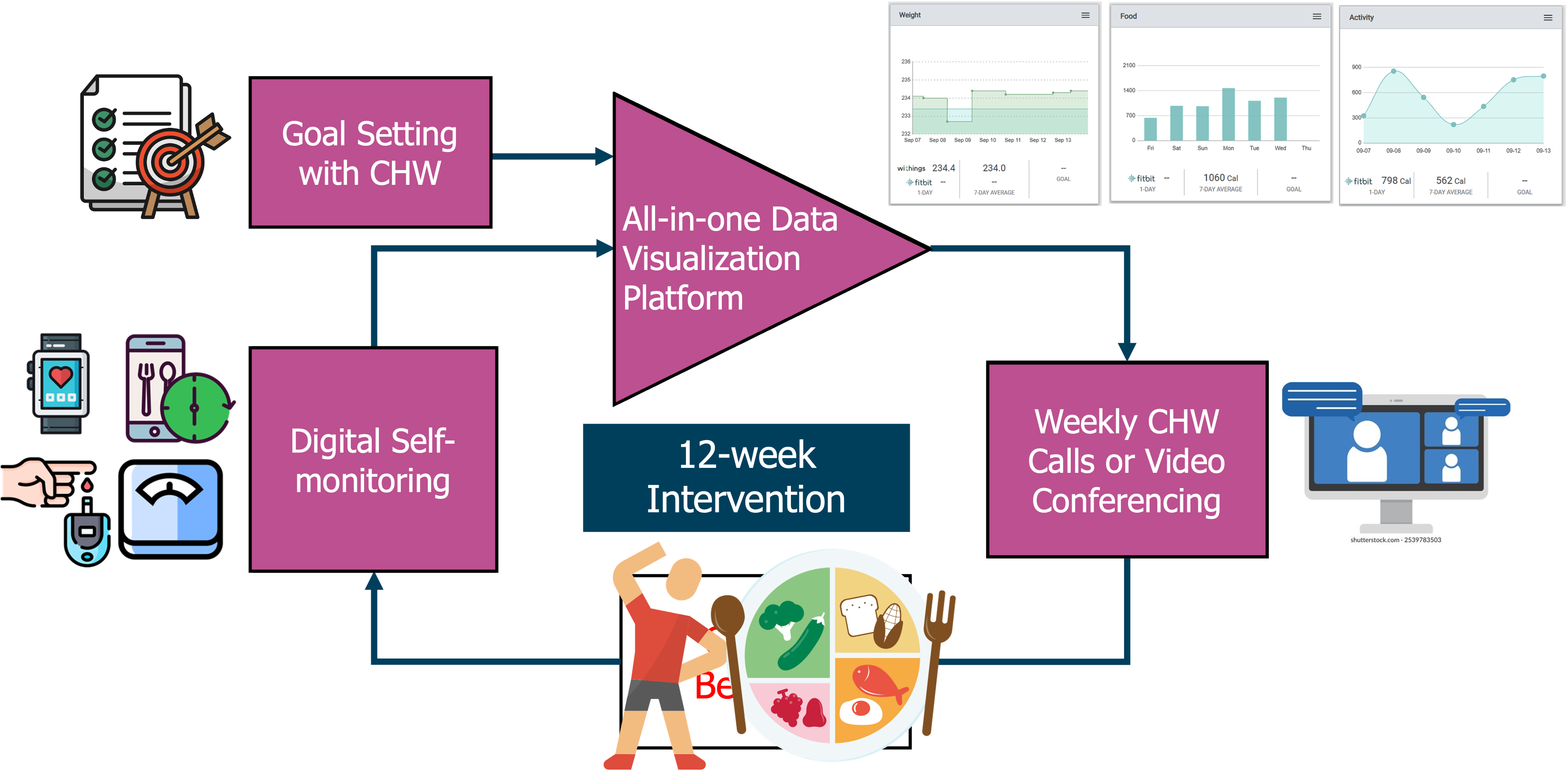
Participants received education on physical activity and massage therapy and were assigned to one of four groups:
|
||
|
Results:
|
||
|
Media Coverage: |
||
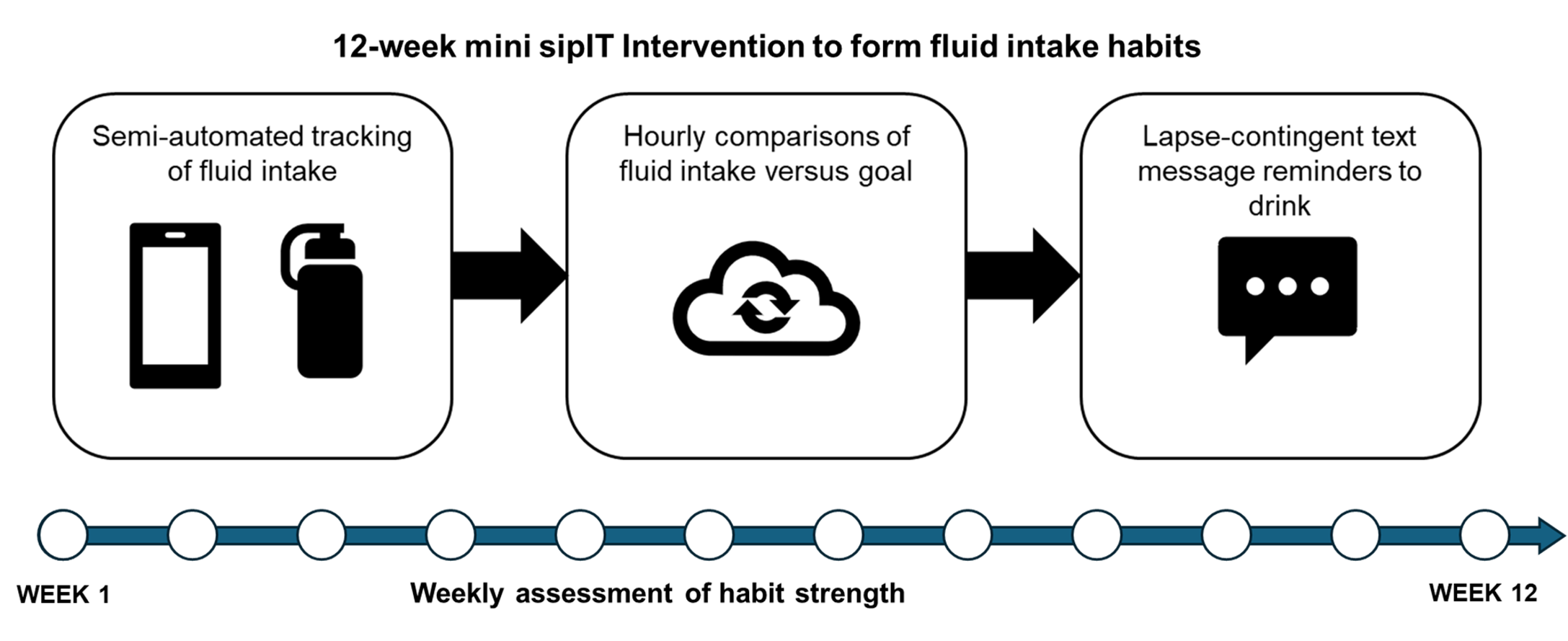
Dual-Process Mechanisms of Action for sipIT Intervention Effects in Patients with Urolithiasis (sipIT Mechanisms Trial) |
||
|
Funder: NIDDK (R01 DK124469) ClinicalTrials.gov ID: NCT06269783 Principal Investigator: David E. Conroy, PhD; Necole Streeper, MD Participants: adults (18+), with recent diagnosis of kidney stone Setting: digital intervention Sample Size: 155 Research Question: How do the reflective and automatic behavioral processes engaged by sipIT change during and after a 3-month intervention period to influence changes in urine output? |
|
|
|
Description: In the 6-month sipIT Mechanisms trial, participants received a three-month just-in-time adaptive intervention (JITAI) designed to promote fluid intake habit formation and prevent kidney stone recurrence.
|
||
|
Results: Data analyses are in progress and will be shared soon. |
||
|
|
||
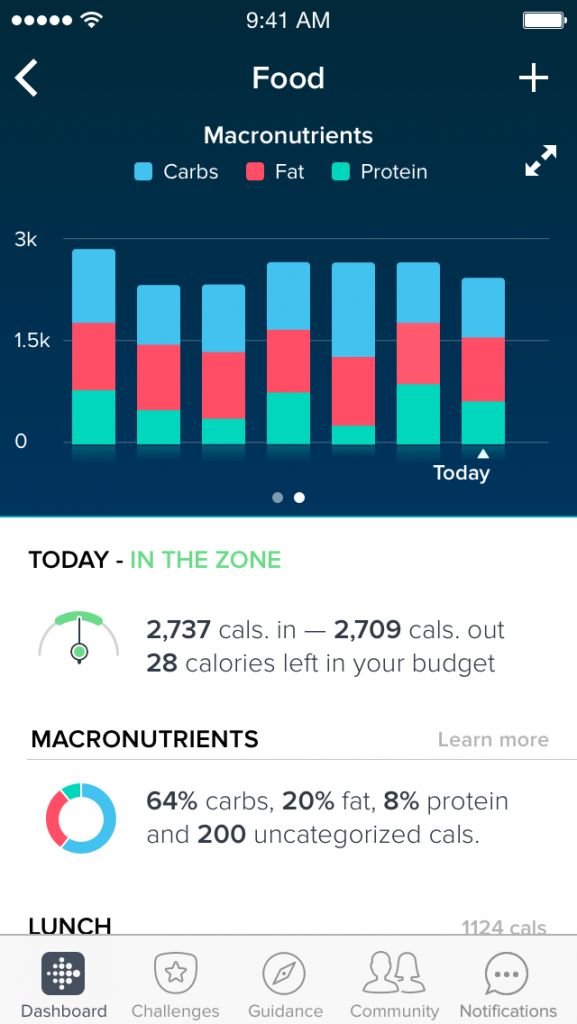
Self-Determination Theory Perspective to Understand Self-Monitoring Trajectories in a Digital Lifestyle Intervention |
||
|
Funder: 80/20 Foundation ClinicalTrials.gov ID: NCT07056335 Principal Investigators: Yan Du, PhD Participants: adults (18+) with overweight/obesity, with or without Type 2 Diabetes or early-stage Chronic Kidney Disease Setting: hybrid Sample Size: 60 Research Question:
|
|
|
|
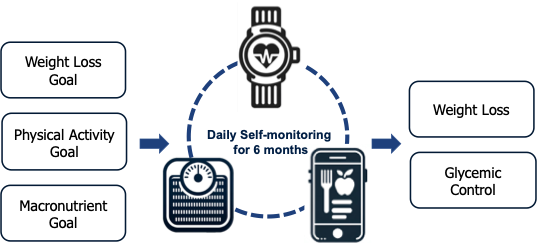
Description: A secondary data analysis of a 6-month stratified, randomized controlled trial. Participants receive instructions to perform daily self-monitoring of diet, physical activity, and body weight for weight loss and diabetes management.
|
||
|
Results:
|
||
Translating Diabetes Self-Management Education and Support Program for Latino Community in Rural South Texas |
||
|
Funder: UT Health San Antonio IIMS/CTSA Principal Investigator: Chun-Fun Chu, PhD Participants: adults (18+), Type 2 Diabetes, Latino, rural south texas residents Setting: hybrid Sample Size: 15 Research Question: To evaluate the feasibility and preliminary efficacy of an mHealth-based DSMES program led by trained community health workers for Latino adults with diabetes living in rural South Texas. |
|
|
|
Description: A 12-week community health worker–led Diabetes Self-Management Education and Support program combining digital education, self-monitoring, and connected technology to support diabetes management in rural Latino communities.
|
||
|
Results:
|
||


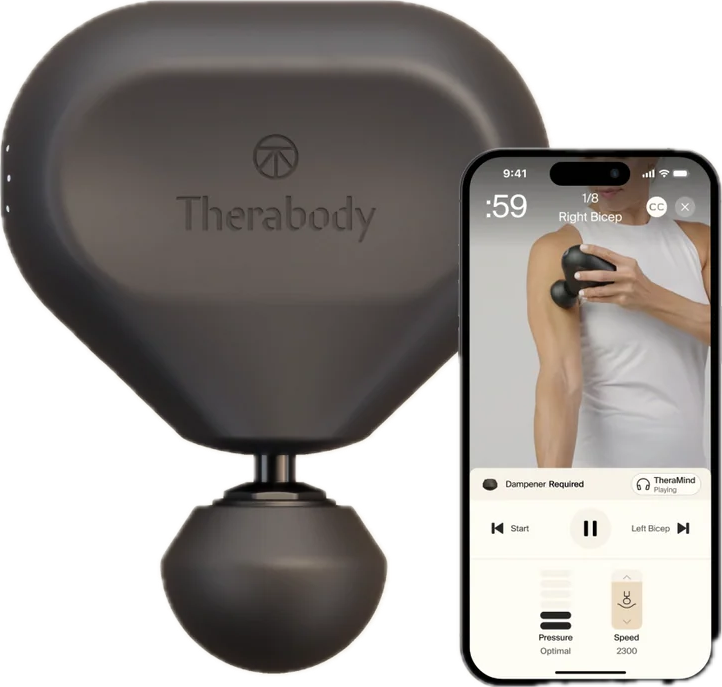 A 2-month 2*2 factorial randomized controlled trial testing whether self-monitoring
physical activity and/or massage gun use improves pain management in older women.
A 2-month 2*2 factorial randomized controlled trial testing whether self-monitoring
physical activity and/or massage gun use improves pain management in older women.
 .
.