Research
Overall Research Goals:
Our laboratory studies how plants and algae acquire CO2 for photosynthesis. One might think that this is a fairly simple process, but a large number of variations in CO2 uptake and acquisition are found in nature. Almost all cyanobacteria and eukaryotic algae have CO2 concentrating mechanisms (CCMs) to increase the concentration of CO2 for fixation. Some higher plants have also developed ways to increase CO2 for photosynthesis. C4 photosynthesis, for example, enables crop plants like corn and sugar cane to concentrate CO2 efficiently.
So why are we interested in how plants and algae acquire CO2 for photosynthesis? In 2011, Zhu, Long and Ort (Ann Rev. Plant Biol. 61:235) pointed out that the efficiency of photosynthetic CO2 fixation is a significant bottleneck to improved crop yield. The FAO report “How to feed the world, 2050” clearly stated that crop yields need to increase to match the growing human population. Our goal is to determine how to improve CO2 fixation in C3 crop plants such as rice and soybean. One of the significant limitations to CO2 fixation in C3 plants is the resistance to CO2 diffusion into mesophyll chloroplasts. Our work on the carbonic anhydrases of Arabidopsis will better our understanding of how CO2 reaches Rubisco for fixation. Our work in Chlamydomonas improves our understanding of which proteins enhance the delivery of CO2 to Rubisco in this alga. The combination of these projects will help scientists better formulate strategies to improve CO2 delivery in crop plants.
We have three main projects in the laboratory. The first project is to study the CCM in Chlamydomonas reinhardtii, a unicellular green alga that is very efficient at pulling CO2 out of the environment. In the second project we are introducing algal CCM components into higher plants to see if they can improve photosynthesis. The third project is to study the role(s) of carbonic anhydrase in the model plant Arabidopsis thaliana.
Further Reading:
Zhu, X.G., Long, S.P. and Ort, D.R. (2010). Improving Photosynthetic Efficiency for Greater Yield. Ann Rev Plant Biol 61:235.
Moroney, J.V., Jungnick, N., DiMario, R.J. and Longstreth, D.J. (2013). Photorespiration and carbon concentrating mechanisms: two adaptations to high O2, low CO2 conditions. Photosyn. Res. 117:121.
Project 1: The CO2 Concentrating mechanism of Chlamydomonas
The first project is the study of the CO2 concentrating mechanism (CCM) of algae. The CCM allows these photosynthetic organisms to concentrate CO2 at the site of Rubisco, increasing photosynthetic efficiency. Rubisco is a very slow enzyme with a low affinity for CO2. Current atmospheric levels of CO2 are less than optimal for Rubisco. So if the CO2 level in a plant can be increased, there is usually an enhancement of photosynthesis. The CCM is very common in nature, with almost all aquatic organisms (algae and photosynthetic bacteria) having some variation of the mechanism. It is estimated that organisms employing a CCM do about 50% of the photosynthesis that takes place on Earth. A well supported model for how the CCM in cyanobacteria works has been developed (Figure 1); however, the CCM is not completely characterized in eukaryotic algae. We have chosen to study the CCM of Chlamydomonas reinhardtii, a unicellular green algae that has been used as a model organism for many years.
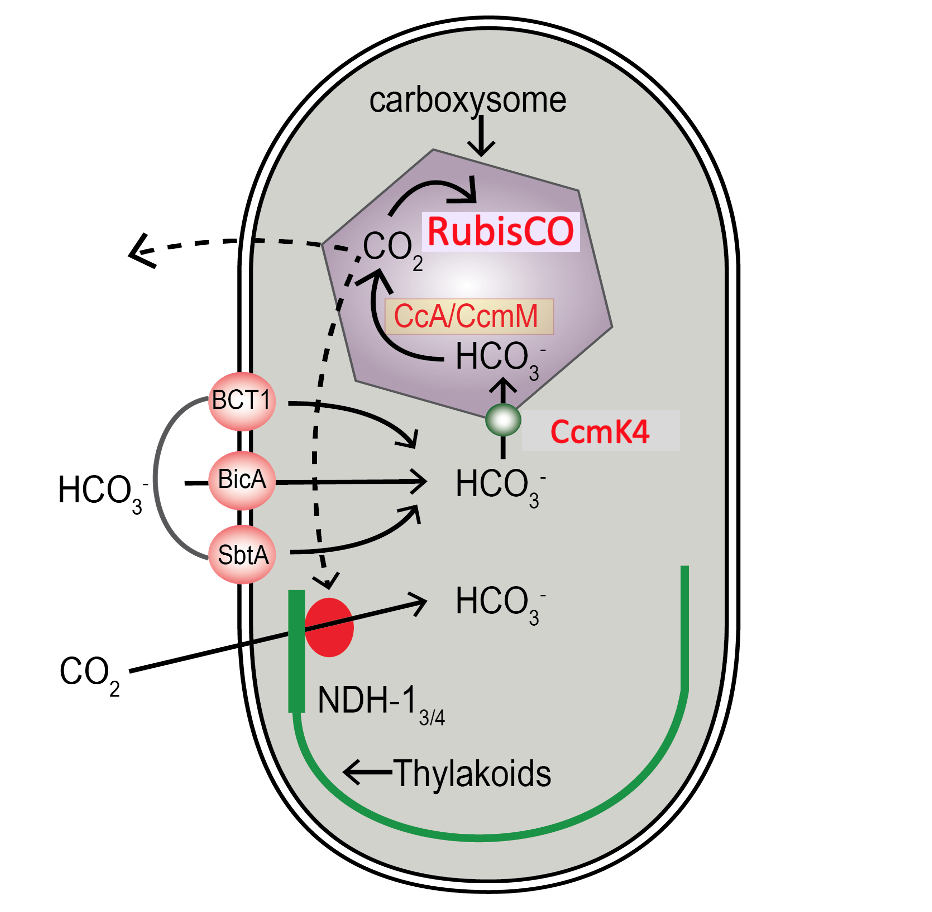
Figure 1
The genome of C. reinhardtii is published (Merchant et al., Science 318: 245). We have used this information and molecular genetics techniques to identify genes encoding carbonic anhydrases, putative bicarbonate transporters and components of the photorespiratory cycle. A few years ago, we initiated an insertional mutagenesis study of the CCM. We have generated and screened 42,000 insertional mutants for clones that could not grow well on low CO2 concentrations. The assumption is that these mutants are defective in a component of the CO2 concentrating mechanism or the photorespiratory cycle. We have identified many mutants from this screen and have been physiologically characterizing these mutants using a combination of genetic complementation, reverse molecular genetics and inverse PCR.
From these studies we have found nine carbonic anhydrase genes and a number of candidate genes that might encode bicarbonate transporters. We have put together a working model of how the CCM works in C. reinhardtii (Figure 2). Currently we are working to localize each carbonic anhydrase within the cells and determine which ones are important in the CCM. We are also working to determine which transporters are actually carrying bicarbonate and whether they are essential to the functioning of the CCM.
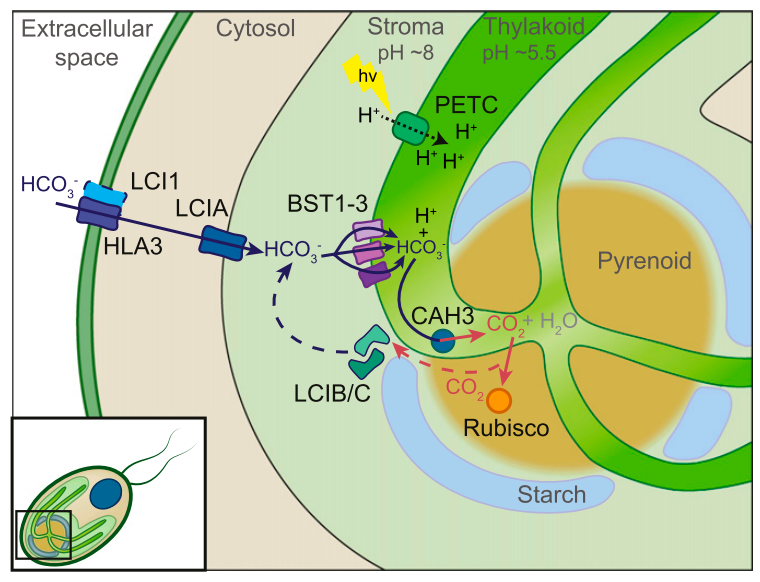
Figure 2
Further Reading:
Rai, A.K., Chen, T., Moroney, J.V. (2021). Mitochondrial carbonic anhydrases are needed for optimal photosynthesis at low CO2 levels in Chlamydomonas. Plant Physiol. kiab351.
Mukherjee, A., Lau, C.S., Walker, C.E., Rai, A.K., Prejean, C.I., Yates, G., Emrich-Mills, T., Lemoine, S.G., Vinyard, D.J., Mackinder, L.C.M., Moroney, J. V. (2019). Thylakoid localized bestrophin-like proteins are essential for the CO2 concentrating mechanism of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 116:16915–16920.
Machingura, M.C., Bajsa-Hirschel, J., Laborde, S.M., Schwartzenburg, J.B., Mukherjee, B., Mukherjee, A., Pollock, S. V, Förster, B., Price, G.D., Moroney, J.V. (2017). Identification and characterization of a solute carrier, CIA8, involved in inorganic carbon acclimation in Chlamydomonas reinhardtii. J. Exp. Bot. 68:3879–3890.
Moroney J.V., Ynalvez R.A. (2007). A proposed carbon dioxide concentration mechanism in Chlamydomonas reinhardtii. Eukaryotic Cell 6:1251.
Merchant et al. (2007). The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 318: 245.
Ma, Y., Pollock, S.V., Xiao, Y., Cunnusamy, K., Moroney J.V. (2011). Identification of a novel gene required for normal pyrenoid formation in Chlamydomonas reinhardtii. Plant Physiol. 156: 884.
Jungnick, N., Ma, Y., Mukherjee, B., Cronan, J.C., Speed, D.J., Laborde, S.M., Longstreth, D.J., Moroney J.V. (2014). The Carbon Concentrating Mechanism in Chlamydomonas reinhardtii – Finding the Missing pieces. Photosynth. Res. 121:159.
Project 2: Increasing photosynthesis in higher plants
The second project is to see if we can improve photosynthesis by placing some of the components of algal CCMs into higher plants. We use model plants such as Arabidopsis and tobacco and then will move forward into crop plants such as rice or cassava. Our research group is part of a large international effort based out of the University of Illinois Campaign-Urbana. This project, named RIPE for Realizing Increased Photosynthetic Efficiency, is funded by the Bill & Melinda Gates Foundation. For more information on the RIPE project go here. Our work centers on utilizing CCM genes from Chlamydomonas and putting those genes into higher plants to improve the delivery of CO2 to Rubisco. We are working closely with Dean Price and Murray Badger at the Australian National University on this project.
Further Reading:
Ort et al. (2015). Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc. Natl. Acad. Sci. USA 112: 8529-8536.
Price et al. (2013). The cyanobacterial CCM as a source of genes for improving photosynthetic CO2 fixation in crop species. J. Exp. Bot. 64:753.
Project 3: The role of carbonic anhydrases in photosynthesis in Arabidopsis
The goal of this project, funded by the National Science Foundation, is to determine which isoforms of the enzyme carbonic anhydrase (CA) are important for photosynthesis in C3 plants. The model C3 plant, Arabidopsis thaliana, has 14 genes that potentially encode CA. We are conducting a molecular characterization of leaf CAs in Arabidopsis. The goals of this work are to determine which CAs are expressed in leaves, which cells express each CA gene, and the intracellular location of each CA isoform. We are also working to obtain knockout Arabidopsis plants missing one or more CA isoforms. We presently have knockout lines for 11 of the 14 CA genes, including all ten genes expressed in leaf tissue. We are constructing double or triple mutants wherever two or more CA isoforms are found in the same location and may have redundant functions. We are also working to determine the physiological roles of the specific CA isoforms by measuring plant growth at different CO2 concentrations and determining mesophyll conductance using gas exchange measurements. We will test the hypothesis that specific CAs are essential to the efficient delivery of CO2 to Rubisco, the enzyme that fixes CO2 in the chloroplast stroma. Knockout plants have been produced and we have found that growth and photosynthesis are reduced in some of these plants. In particular, the double mutant missing two cytosolic CAs, βCA2 and βCA4, has reduced growth and an imbalance in its amino acid pools (Dimario et al. Plant Physiol 171:280). While the large number of CA isoforms in Arabidopsis makes the untangling of their physiological roles complex, the sequenced Arabidopsis genome and the availability of knockout lines for most of the CA genes make the project quite feasible.
Further Reading:
DiMario, R.J., Machingura, M.C., Waldrop, G.L., Moroney, J.V. (2018). The many types of carbonic anhydrases in photosynthetic organisms. Plant Sci. 268:11–17.
DiMario, R.J., Quebedeaux, J.C., Longstreth, D.J., Dassanayaki, M., Hartman, M.M., Moroney, J.V. (2016). βCA2 and βCA4 are required for optimal plant growth in a low CO2 environment. Plant Physiol. 171: 280.
DiMario, R.J., Clayton, H., Mukherjee, A., Ludwig, M., Moroney, J.V. (2016). Plant carbonic anhydrases – structures, locations, evolution and physiological roles. Mol. Plant 10(1):30-46.