Research
Carborane-functionalized porphyrin derivatives
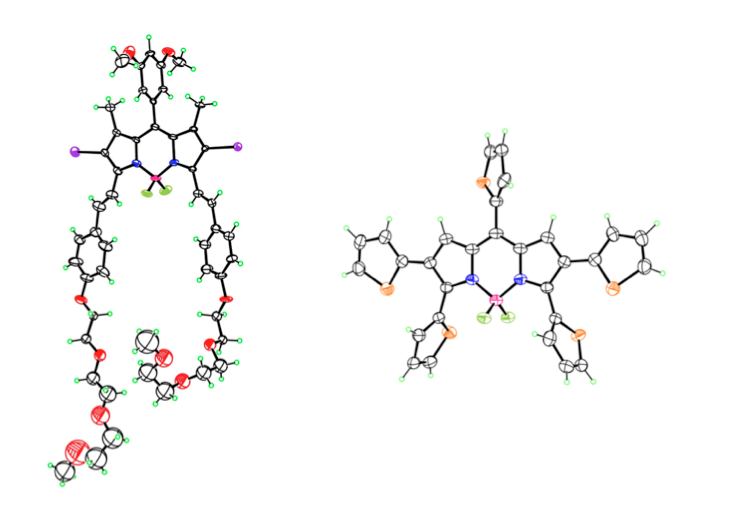
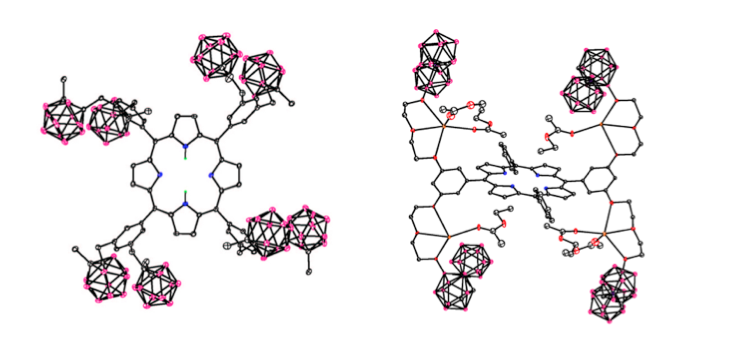
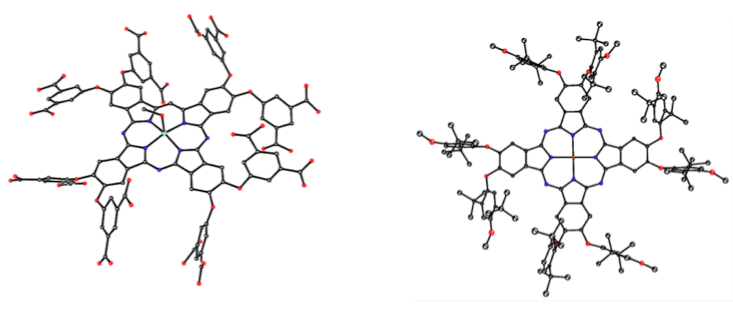
Our research in this area has been funded by NIH (R01 CA098902) and centers on the synthesis, chemical characterization and biological evaluation of carborane-containing porphyrins and their derivatives for application as boron delivery agents for boron neutron capture therapy (BNCT). Our targets are high-grade gliomas and a variety of extracranial tumors, including inoperable head and neck cancers, for which there is currently no chemotherapy available. BNCT is based on the nuclear reaction that occurs when boron-10 nuclei, localized within tumor cells, capture low-energy neutrons to produce high linear energy transfer particles. Our work in this area has shown that carboranylporphyrins bearing stable carbon-carbon bonds between the macrocycle and boron clusters are amongst the most promising boron delivery agents; this is because they are non-toxic, fluorescent, have long retention times in tumors and can deliver therapeutic amounts of boron to tumor-bearing animals by convection enhanced delivery.
Representative publications in this area:
- R. F. Barth, J. A. Coderre, M. G. H. Vicente and T. E Blue. Boron Neutron Capture Therapy of Cancer: Current Status and Future Prospects. Clinical Cancer Research 2005, 11, 3987-4002.
- M. Sibrian-Vazquez, E. Hao, T. J. Jensen and M. G. H. Vicente. Enhanced Cellular Uptake with a Cobaltacarborane-Porphyrin-HIV-1 Tat 48-60 Conjugate. Bioconjugate Chemistry 2006, 17, 928-934.
- S. Kawabata, W. Yang, R. F. Barth, G. Wu, T. Huo, P. J. Binns, K. J. Riley, O. Ongayi, V. Gottumukkala and M. G. H. Vicente. Convection Enhanced Delivery of Carboranylporphyrins for Neutron Capture Therapy of Brain Tumors. Journal of Neuro-Oncology 2011, 103, 175-185.
- N. V. S. D. K. Bhupathiraju, X. Hu, Z. Zhou, F. R. Fronczek, P.-O. Couraud, I. A. Romero, B. Weksler, and M. G. H. Vicente. Synthesis and in Vitro Evaluation of BBB Permeability, Tumor Cell Uptake, and Cytotoxicity of a Series of Carboranylporphyrin Conjugates. Journal of Medicinal Chemistry, 2014, 57, 6718–6728.
Near-IR photosensitizers and their conjugates
This research has been funded by NIH (R21 CA139385 and R01 CA179902) and NSF CRC-304833. Our research in this area involves the synthesis and development of near-IR photosensitizers, based on phthalocyanine, tetrabenzoporphyrin and chlorin macrocycles, for applications as biological labels, and as sensitizers for the detection and treatment of cancers by photodynamic therapy (PDT). We investigate the structural, photophysical and chemical properties of the photosensitizers and develop methodology for their conjugation to various molecules, including peptides, proteins and monoclonal antibodies. Some of our conjugates are designed to target specific receptors, including the epidermal growth factor (EGF) receptors and the vascular endothelial growth factor (VEGF) receptors. We develop conjugation techniques, and evaluate the biological properties of our photosensitizers in cells in culture and in animal models. We have observed up to 37-fold increases in cancer cell targeting-ability for certain conjugates compared with untargeted macrocycles.
Representative publications in this area:
- W. Liu, T. J. Jensen, F. R. Fronczek, R. P. Hammer, K. M. Smith and M. G. H. Vicente. Synthesis and in vitro Studies of Non-aggregated Water-soluble Phthalocyanines. Journal of Medicinal Chemistry 2005, 48, 1033-1041.
- M. Sibrian-Vazquez, T. J. Jensen, R. P. Hammer and M. G. H. Vicente. Peptide-mediated Cell Transport of Water Soluble Porphyrin Conjugates. Journal of Medicinal Chemistry 2006, 49, 1364-1372.
- M. Sibrian-Vazquez, X. Hu, T. J. Jensen and M. G. H. Vicente. Synthesis and cellular studies of PPIX-homing peptide conjugates. Journal of Porphyrins and Phthalocyanines 2012, 16, 603-615.
- I. Sehgal, H. Li, B. G. Ongarora, D. Devillier and M. G. H. Vicente. Synthesis and biological investigations of a ZnPc-antiCEA bioconjugate for imaging of colorectal cancer. Journal of Porphyrins and Phthalocyanines 2013, 17, 150-156.
BODIPY-based fluorophores for bioimaging
Our research in this area has been funded by NSF (CHE-1362641, CHE-0911629 and CHE-1800126). This project involves the design, synthesis, and investigation of boron dipyrromethene (BODIPY)-based dyes for applications in bioimaging and biosensing. This type of dye displays a rich array of photophysical and optoelectronic properties, including intense absorption and emission profiles, high fluorescence quantum yields, and long fluorescence lifetimes. We have been particularly interested in developing new synthetic routes to near-IR absorbing and emitting BODIPY dyes of high chemical- and photo-stability as well as aqueous solubility. We investigate conjugation methodologies of the BODIPYs to biomolecules, and evaluate their photophysical, chemical and biological properties.
Representative publications in this area:
- L. Jiao, J. Li, S. Zhang, C. Wei, E. Hao and M. G. H. Vicente. A Selective Fluorescent Sensor for Imaging Cu2+ in Living Cells. New Journal of Chemistry 2009, 33, 1888-1893.
- T. Uppal, X. Hu, F. R. Fronczek, S. Maschek, P. Bobadova-Parvanova, and M. G. H. Vicente. Synthesis, Computational Modelling and Properties of Benzo-appended BODIPYs. Chemistry – A European Journal, 2012, 18, 3893-3905.
- T. Uppal, N. V. S. D. K. Bhupathiraju and M. G. H. Vicente. Syntheses and Cellular Properties of Near-IR BODIPY-PEG and Carbohydrate Conjugates. Tetrahedron 2013, 69, 4687-4693.
- J. H. Gibbs, Z. Zhou, D. Kessel, F. R. Fronczek, S. Pakhomova, and M. G. H. Vicente. Synthesis, Spectroscopic, and in vitro Investigations of 2,6-Diiodo-BODIPYs with PDT and Multi-mode Imaging Applications. J. Photochemistry Photobiology B: Biology 2015, 145, 35-47.