Research
Chronic wound infections are complicated by the formation of bacterial biofilms, commonly by Pseudomonas aeruginosa, Acinetobacter baumannii, Escherichia spp. and Staphylococcus aureus. Biofilm embedded cells can be up to 1000-fold more tolerant to antibiotic treatment than free living (planktonic) cells. Antibiotic tolerance, a condition which does not involve mutation, enables bacteria to survive in the presence of antibiotics. Hence, the antibiotic tolerance of biofilm-cells often renders antibiotics ineffective, even against strains that do not carry resistance-impairing mutations. The antibiotic tolerance of biofilm bacteria is compounded by the intrinsic antibiotic resistance of multidrug resistant strains. Consequently, there is an urgent need to explore and discover new strategies to kill biofilm-embedded cells to combat biofilm infections.
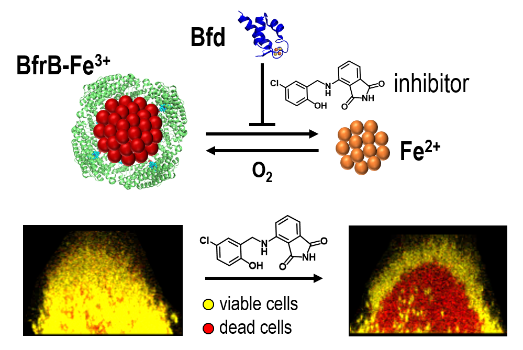
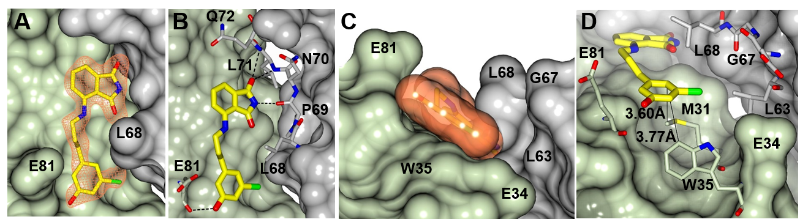
Pathogens depend strongly on well-regulated iron homeostasis to counteract the strict iron limitation imposed by the host immune response. To disrupt iron homeostasis, we are targeting the iron storage protein bacterioferritin (BfrB) and its physiological partner Bfd, which are present only in bacteria. We have shown that BfrB regulates cytosolic iron concentrations by (i) oxidizing Fe2+ and storing up to ~3,000 Fe3+ atoms in its internal cavity, and (ii) forming a complex with the electron transfer protein Bfd, to reduce Fe3+ in the internal cavity of BfrB and mobilize Fe2+ into the cytosol for its incorporation in metabolism. We demonstrated that blocking the BfrB-Bfd complex in P. aeruginosa cells by deletion of the bfd gene induces iron homeostasis dysregulation by causing an irreversible accumulation of Fe3+ in BfrB and concomitant iron deprivation in the cytosol, which leads to impaired biofilm maintenance and biofilm cell death. We also discovered proof-of-concept inhibitors of the BfrB-Bfd complex, which function by binding to BfrB at the Bfd-binding site. Our work showed that the small molecule inhibitors of the BfrB-Bfd complex bind BfrB in the P. aeruginosa cytosol, inhibit iron mobilization and elicit cell death in the biofilm. We are currently pursuing work to evolve the proof-of-concept inhibitors of the BfrB-Bfd complex into: (a) lead compounds for developing antibiofilm therapies and (b) probe-molecules for investigating bacterial iron homeostasis and discovering new connections between bacterial iron management and bacterial metabolism.
(ACS Infect. Dis. 2021, 7, 123-140)