Research Interests
Welcome to the Ragains Group!
We are a group of synthetic organic chemists with broad research interests that include synthetic method development, total synthesis of natural products, carbohydrate chemistry, photochemistry, and surface chemistry. We have had members at various stages of their careers including Ph.D. students, postdocs, undergraduates, and even high school students. If you are interested in pursuing graduate studies in my group, please feel free to contact me at my email (jragains@lsu.edu)! However, you should also make a point of visiting our departmental web page that describes the application process for the Department of Chemistry's graduate studies program.
In addition to this, you should contact Dr. Caroline Schneider at gradchem@lsu.edu and you are likely to have any questions you might have about the application process answered. If you are interested in postdoctoral work or undergraduate research, please contact me at the email mentioned above.
As mentioned earlier, we have a number of research interests.
Synthetic Method Development/Carbohydrate Chemistry/Photochemistry
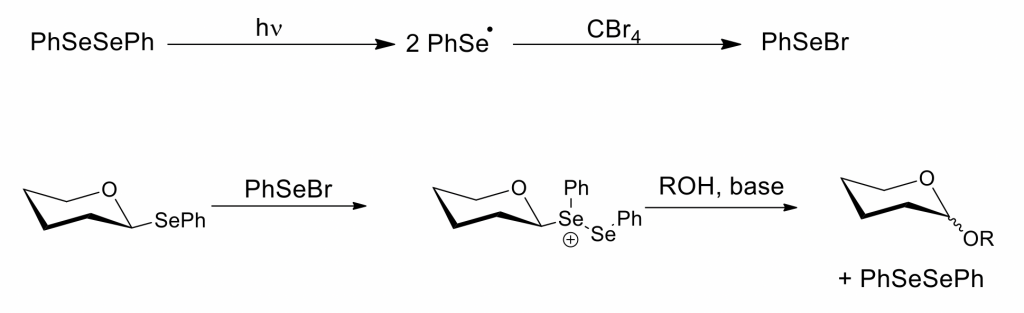
For several years now, we have been interested in the development of synthetic methods that harness energy from visible light. In particular, we have developed O-glycosylation reactions that involve the visible-light irradiation of diphenyldiselenide and carbon tetrabromide in the presence of selenoglycoside electrophiles and alcohol nucleophiles (Carbohydr. Res. 2013,67, 42-47). The method is marked by mildness and the simplicity of the reaction setup, and the mechanism that we proposed involved radical intermediates and the selenium electrophile PhSeBr:
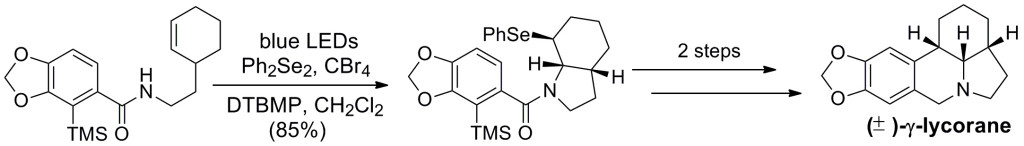
Our continuing work in this area was applied toward a selenofunctionalization reaction that was leveraged toward the synthesis of the alkaloid γ-lycorane (Org. Lett. 2013, 15, 5558-5561):
More recently, our efforts in the area of visible-light photochemistry have led us to a novel method for the remote functionalization of C-H bonds in aryl alkyl sulfones (Org. Lett. 2017,19, 5553-5556). Here, blue LED irradiation of mixtures of o-diazoniaphenyl alkyl sulfones, the visible-light photocatalyst Ru(bpy)32+, and various nucleophiles have led to the formation of C-H functionalized compounds resulting from remote functionalization by way of hydrogen atom transfer to an intermediate aryl radical and polar crossover to carbocation that then intercepts nucleophile:
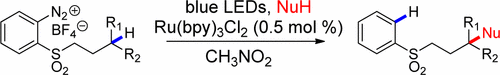
We have also published a related remote hydroxylation/etherification method that did not require visible-light irradiation (Angew. Chem. Int. Ed. 2015,54, 7837-7841).
In addition, we have developed an O-glycosylation protocol involving the visible-light irradiation of 4-(4-methoxyphenyl)-3-butenylthioglycosides in the presence of Umemoto's reagent (Angew. Chem. Int. Ed. 2016, 55, 6515-6519). This method of activation is orthogonal to other classes of thioglycoside, and the mechanism that we proposed is somewhat unusual, involving the intermediacy of an electron donor-acceptor (EDA) complex. Evidence for the EDA complex and its importance as an intermediate in the O-glycosylation reaction was gained through spectroscopic investigations in addition to theoretical work done in collaboration with Prof. Revati Kumar (LSU Dept. of Chemistry):
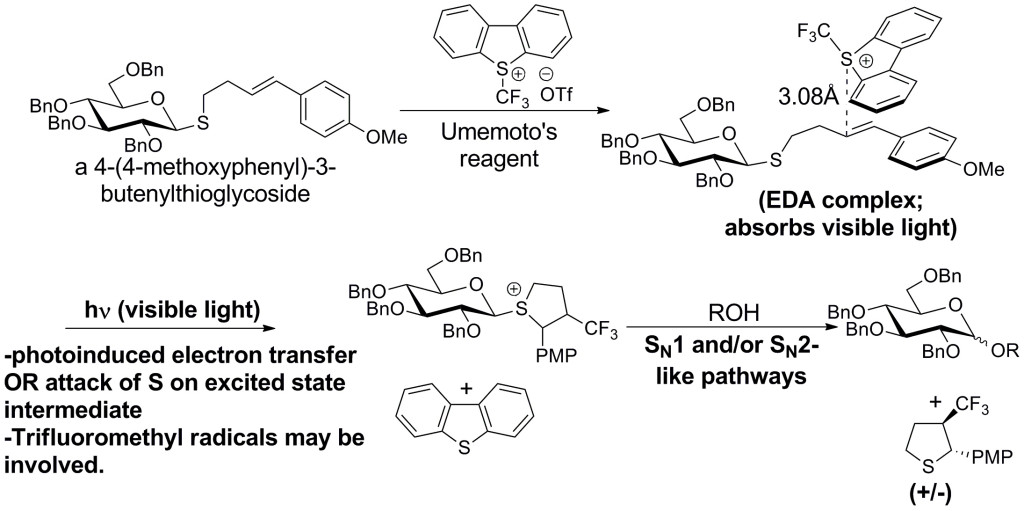
Our continuing efforts in the area of O-glycosylation are central to method development in our group, and newly developed methods will be applied toward the synthesis of naturally occurring oligosaccharides and other synthetic targets.
Total Synthesis of Natural Products
In addition to the previously mentioned efforts culminating in the synthesis of γ-lycorane, multistep synthesis of other natural products constitutes a longstanding area of interest in our lab. We have synthesized a series of 3,6-dideoxyglycosides known as "ascarosides." These compounds are constitutively produced by nematodes (unsegmented roundworms) including the model organism Caenorhabditis elegans and the insecticidal nematode Heterorhabditis bacteriophora. Our collaborators Rebecca Butcher (Dept. of Chemistry, Univ. of Florida) and Michael Miller (Dept. of Developmental Biology, University of Alabama-Birmingham) have elegantly demonstrated the importance of these compounds in such diverse events as fertilization (Science 2014, 344, 754-757)and diapause (ACS Chem. Biol. 2012,7, 961-966 and Bioorg. Med. Chem. 2013, 21, 5754-5769)in nematodes using our synthetic ascarosides, and their findings have implications in deepening our understanding of the biology of higher organisms such as Homo sapiens.
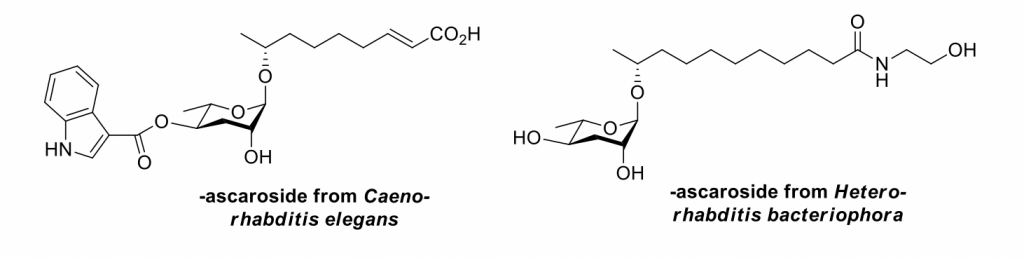
Our continuing efforts in the area of natural product synthesis are geared toward the synthesis of bacterial oligosaccharides. While chemoenzymatic methods developed elsewhere have made great strides toward the production of serviceable quantities of mammalian oligosaccharides, biosynthetic machinery for the production of bacterial oligosaccharides and neoglycosides is much more difficult to harness. As such, multistep organic synthesis will remain an important tool in the production of oligosaccharides for the foreseeable future. Specifically, we will leverage O-glycosylation and other methods developed in our group toward the synthesis of biologically relevant oligosaccharides.
Surface Chemistry
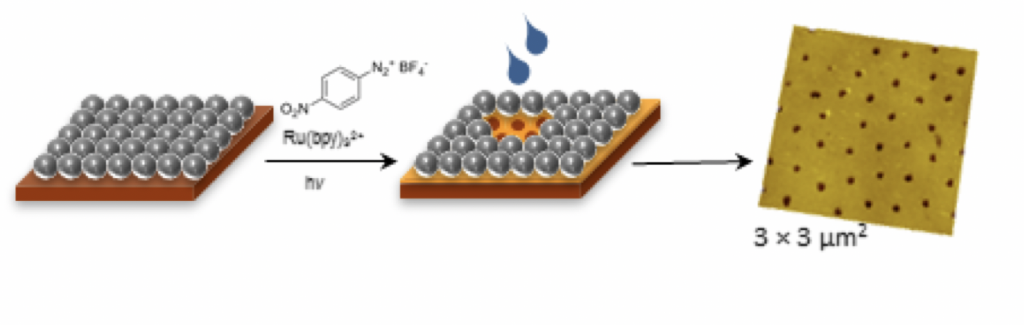
Our interests in visible-light photochemistry and, specifically, the generation of carbon-centered radicals using visible-light photocatalysis have led us quite unexpectedly into the area of surface chemistry. In collaboration with the laboratory of Prof. Jayne Garno (LSU Dept. of Chemistry), we have developed a series of nanolithography methods that we refer to as "visible-light photocatalytic particle lithography." Our initial efforts (J. Am. Chem. Soc. 2014, 136, 14438-14444) were geared toward the deposition of nanostructured thin films on gold via the generation of aryl radicals with the visible-light irradiation of Ru(bpy)32+in the presence of aryldiazonium tetrafluoroborate salts. More recently, we have extended this method to redox-active esters as precursors to alkyl radicals for thin film formation (Beilstein J. Nanotechnol. 2017, 8, 1863-1877). While the mechanism of thin film deposition is a topic of some dispute, attachment of radicals by covalent bonding to the Au surface followed by oligomerization and crosslinking processes are likely to contribute to the formation of robust organic thin films. The resulting thin films could have applications in the development of fouling- and corrosion-resistant surfaces and the development of biosensors. In the cartoon below, a gold substrate covered with silicon dioxide mesospheres (left) is irradiated in the presence of an arenediazonium tetrafluoroborate salt and Ru(bpy)32+. Aryl radicals formed under the conditions attach to the surface and generate a thin film in the spaces not masked by the mesospheres. The mesospheres are then washed away (middle) revealing the nanostructured thin film of which an atomic force microscope image is shown at right.