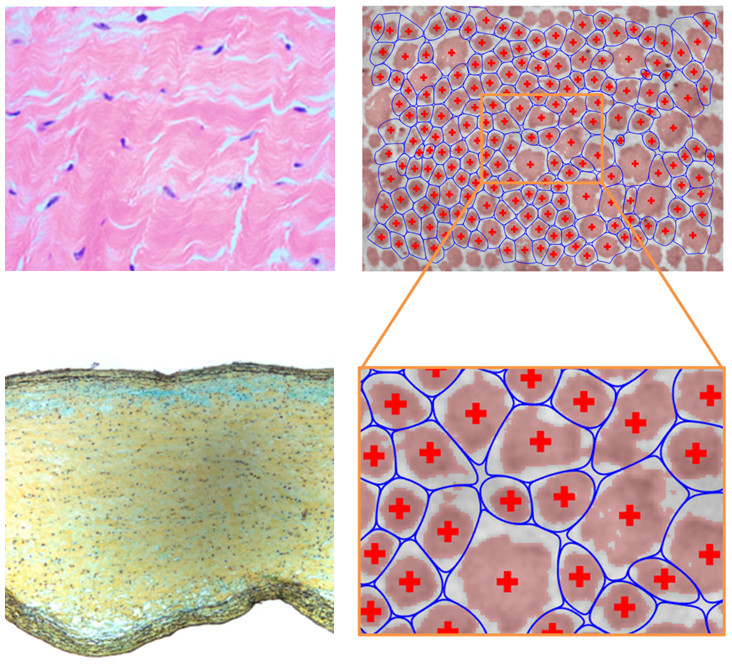
Experiments
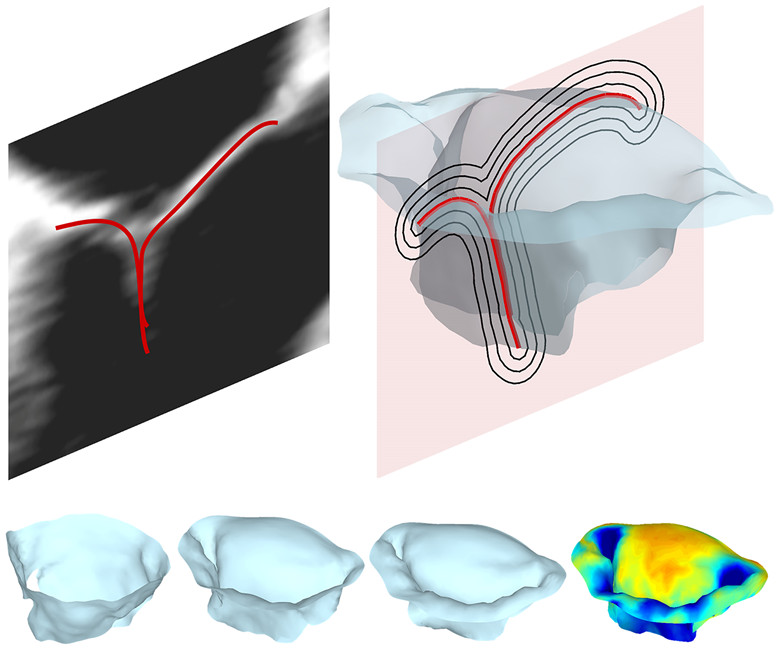
Modeling
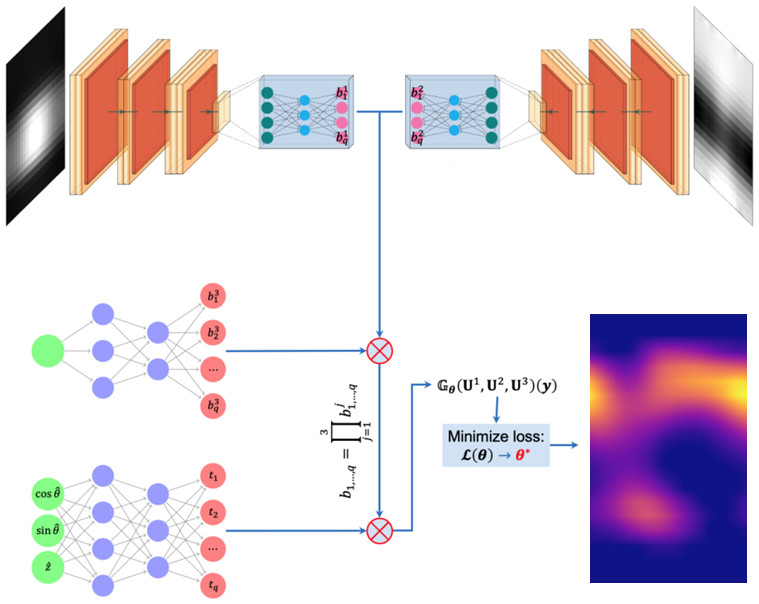
Computation
Overview of the Lab
Clinical motivation
Cardiovascular tissues bear dynamic loads and must adapt to preserve their function, a process continually governed by tissue-resident cells and influenced by immune cell infiltration. While progress has been made over recent decades to better understand the biomechanical and mechanobiological mechanisms underpinning the maintenance and adaptation of cardiovascular tissues, long-term clinical outcomes of disease (e.g., myocardial infarction, congenital defects, aneurysms/dissections) and therapies (e.g., surgery, medication) remain difficult to predict. It is thus vital to further elucidate how the structure and function of cardiovascular tissues are mediated via mechanotransduction and subsequent remodeling of the extracellular matrix in order to promote homeostasis or understand its loss. To improve prognostic predictions and therapeutic outcomes for patients with pathologies that involve multiple tissue types (e.g., infarction coincident with mitral regurgitation, bicuspid aortic valve coincident with aortic aneurysm), there is also a critical need to model subsets of the cardiovascular system as unified biomechanical units.
What we do
At the Cardiovascular Engineering Lab (CEL), we use engineering analysis and design
principles to develop novel approaches for understanding the regulation and adaptation
of cardiovascular tissues under non-pathological and pathological conditions alike.
As an end goal, we emphasize the development of patient-specific computational and
mathematical models that can predict clinically relevant outcomes in order to enable
the optimization and personalization of therapeutic interventions. These models are
informed by in vivo images of the cardiovascular system as well as experimental investigations of the
mechanical properties, composition, microstructure, and cell biology of cardiovascular
tissues. Our work spans the organ, tissue, cellular, and subcellular scales, with
a focus on elucidating (1) the functional biomechanical interactions between coupled
myocardial, valvular, and vascular tissues, and (2) the mechanobiological interplay
between the tissue and cellular scales that together govern the progression of disease
as well as remodeling in response to therapies.