Depot-specific regulation of adipogenesis
Two most important white fat depots of mammals are visceral fat depot and subcutaneous fat. Recent studies showed that the impact of adipose tissue expansion on human health dependent on fat depots. People with high visceral fat content are more prone to diabetes and other metabolic disorders than those with more abundant subcutaneous fat.
In addition, intramuscular fat depot, an important fat depot in beef industry, is another major white fat depot. In human, intramuscular fat accumulation is a typical symptom of muscular dystrophies, which greatly affects muscle function. In beef cattle, adipogenesis at intramuscular fat depot is responsible for marbling, which is highly desired due to its positive influence on meat quality. The grade of marbling is highly variable between different cattle breeds. Two good examples are Wagyu cattle and Brahman cattle which are known for abundant marbling and extreme low marbling respectively. However, subcutaneous fat accumulation in Wagyu cattle and Brahman cattle are overall similar.
All of these suggest the presence of depot-specific regulation of adipogenesis. Currently, we are using both cattle and mouse as models to study the mechanism regulating adipogenesis at different fat depots.
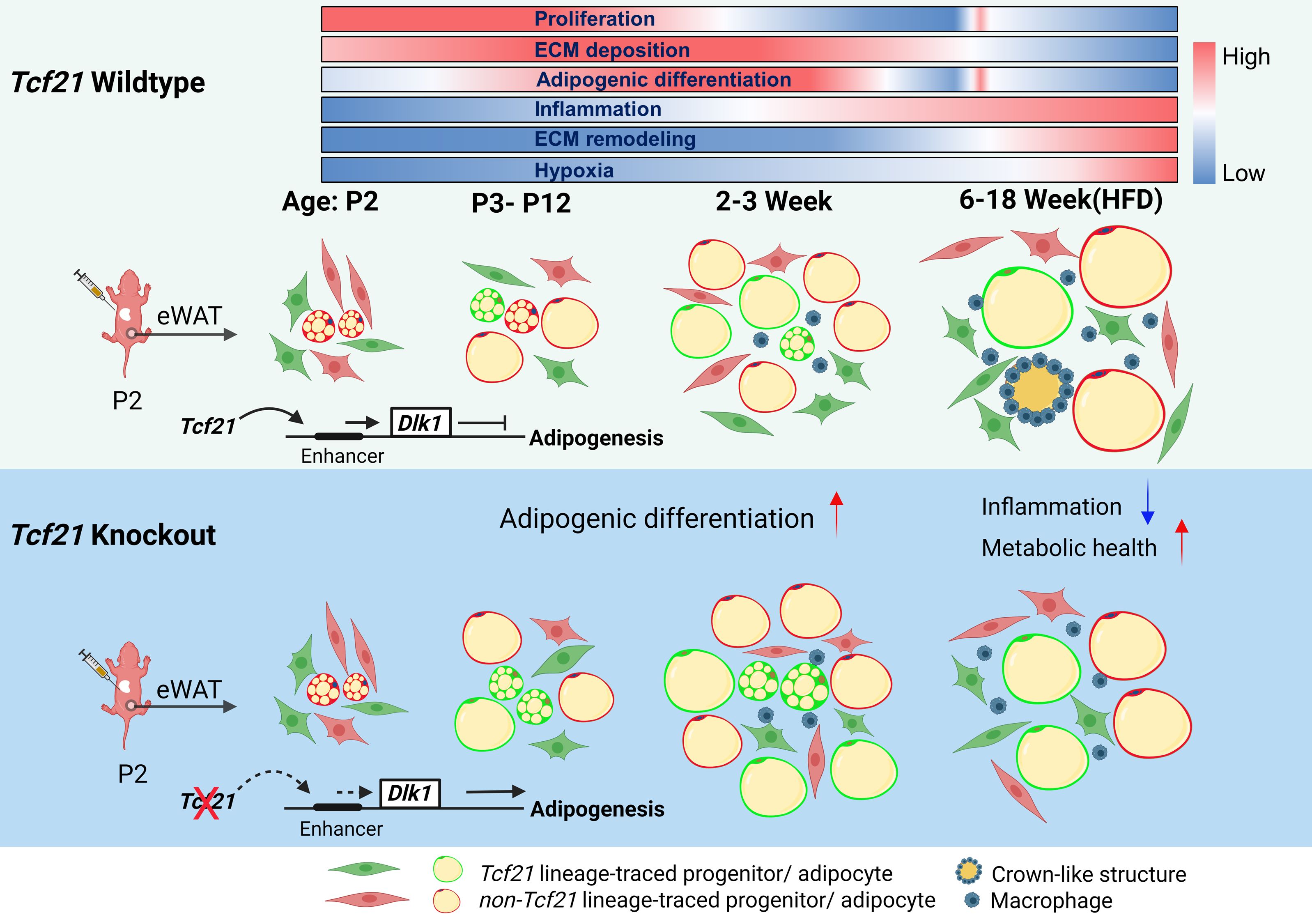
Distinct locations of different white adipose depots suggest anatomy-specific developmental regulation, a relatively understudied concept. Here, we report a population of Tcf21 lineage cells (Tcf21 LCs) present exclusively in visceral adipose tissue (VAT) that dynamically contributes to VAT development and expansion. During development, the Tcf21 lineage gives rise to adipocytes. In adult mice, Tcf21 LCs transform into a fibrotic or quiescent state. Multiomics analyses show consistent gene expression and chromatin accessibility changes in Tcf21 LC, based on which we constructed a gene-regulatory network governing Tcf21 LC activities. Furthermore, single-cell RNA sequencing (scRNA-seq) identifies the heterogeneity of Tcf21 LCs. Loss of Tcf21 promotes the adipogenesis and developmental progress of Tcf21 LCs, leading to improved metabolic health in the context of diet-induced obesity. Mechanistic studies show that the inhibitory effect of Tcf21 on adipogenesis is at least partially mediated via Dlk1 expression accentuation.
Article
- Tcf21 marks visceral adipose mesenchymal progenitors and functions as a rate-limiting factor during visceral adipose tissue development (ScienceDirect.com)
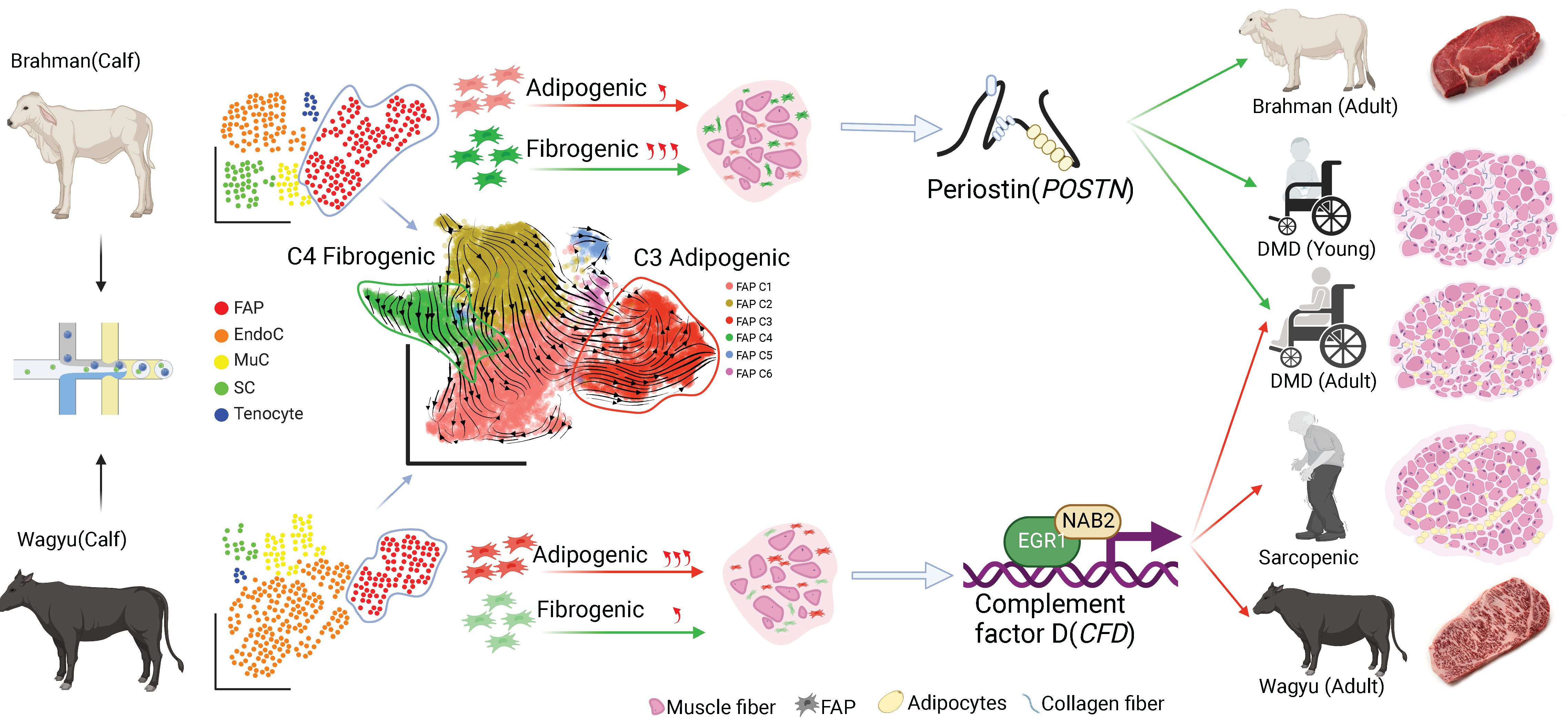
Intramuscular fat (IMF) and intramuscular connective tissue (IMC) are often seen in human myopathies and are central to beef quality. The mechanisms regulating their accumulation remain poorly understood. Skeletal muscle single-cell RNAseq was performed on three cattle breeds, including Wagyu (high IMF), Brahman (abundant IMC but scarce IMF), and Wagyu/Brahman cross. A total of 32,708 cells and 21 clusters were identified, including fibro/adipogenic progenitor (FAP) and other resident and infiltrating cell types. We identified an endomysial adipogenic FAP subpopulation enriched for COL4A1 and CFD (log2FC=3.19 and 1.92, respectively; p<0.0001) and a perimysial fibrogenic FAP subpopulation enriched for COL1A1 and POSTN (log2FC=1.83 and 0.87, respectively; p<0.0001), both of which were likely derived from an unspecified subpopulation. Further analysis revealed more progressed adipogenic programming of Wagyu FAPs and more advanced fibrogenic programming of Brahman FAPs. Mechanistically, NAB2 drives CFD expression, which in turn promotes adipogenesis. CFD expression in FAPs of young cattle before the onset of intramuscular adipogenesis was predictive of IMF contents in adulthood (R2=0.885, p<0.01). Similar adipogenic and fibrogenic FAPs were identified in humans and monkeys. In aged humans with metabolic syndrome and progressed Duchenne muscular dystrophy (DMD) patients, increased CFD expression was observed (p<0.05 and p<0.0001, respectively), which was positively correlated with adipogenic marker expression, including ADIPOQ (R2=0.303, p<0.01; and R2=0.348, p<0.01, respectively). The specificity of Postn/POSTN as a fibrogenic FAP marker was validated using a lineage-tracing mouse line. POSTN expression was elevated in Brahman FAPs (p<0.0001) and DMD patients (p<0.01) but not in aged humans. Strong interactions between vascular cells and FAPs were also identified.