Home
Supramolecular metal-organic compounds
Supramolecular chemistry deals with the assembly of large structures from small building blocks, and the goal is to find large structures that have unusual properties or reactivity. In our work, the building blocks are metal ions and multifunctional organic ligands. What kinds of large structures form when they are mixed? Will other “guest” molecules fit inside the supramolecular “host” compounds after they form? What kind of reactions will they undergo?
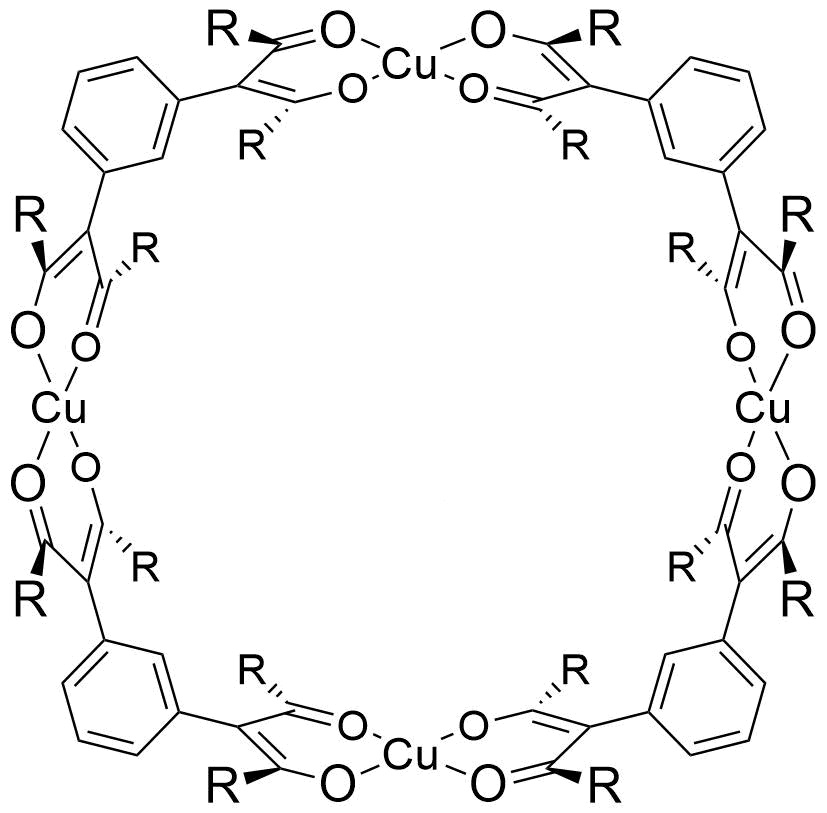
β-Diketonate molecular squares
The bis(β-diketone) m-pbprH2 reacts with Cu2+ to produce a “molecular square”, Cu4(m-pbpr)4. This molecule (shown below at left) is about 14 Å across, just the right size to fit buckminsterfullerene (C60). The result is the host-guest adduct Cu4(m-pbpr)4(μ-C60). You can view the structure of the molecule in 3D by clicking on the link below the picture.
Click here to view molecular square in 3D (in a new window)
Polyhedral complexes from tris(β-diketones)
If a bis(β-diketone) will make a polygon-shaped (square-shaped) molecule, what about a tris(β-diketone)? We wished to determine whether metal-organic polyhedra (i.e. molecules with 3D arrangements of metals and ligands) could be prepared from tris(β-diketones). These 3D metal-organic polyhedra (MOPs) could be as much as ~50 Å in diameter. We have prepared the proposed Cu(II) tris(β-diketonates), but their characterization is challenging. Data from atomic force microscopy (AFM) and analytical ultracentrifugation (AUC) experiments suggest that the main product from reaction of MeSi(phacH)3 with Cu2+is a much smaller molecule than expected (diameter < 20 Å; MW < 1000). Thanks to Professors Paul Russo & Jayne Garno for assistance with these experiments.
Redox-active complexes of pyridyltriazole ligands
Nitrogen-containing ligands are good choices if you want to be able to oxidize or reduce metal complexes. We have used pyridyltriazoles to make supramolecular metal complexes with Cu, Ni, and Pd.
With m-xpt, a bis(pyridyltriazole) ligand, we make an “empty” copper(II) complex, [Cu2(m-xpt)2]4+, which is blue in solution. The blue Cu(II) complex is reduced to yellow Cu(I) by treatment with sodium ascorbate (a salt of vitamin C). This Cu(I) dimer
changes into a green Cu(II) species that contains a bridging oxalate ion (C2O42−). At first, we thought this reaction involved reduction of CO2 from the air. However, we now know that the oxidation actually requires O2, and the oxalate comes from oxidation of ascorbate.