Effects of Oil and Chemical Responses on Fresh Marsh Function and Oil Degradation: Response Implications
John Andrew Nyman, Ph.D.
William H. Patrick, Jr., Ph.D.
University of Southwestern Louisiana
Louisiana State University
Technical Report Series
95-011
DISCLAIMER
This report was prepared under contract between John A. Nyman and the Louisiana Oil Spill Coordinators Office/Office of the Governor, Louisiana Applied Oil Spill Research and Development Program. The contents of this document do not necessarily reflect the views and policies of the Louisiana Oil Spill Coordinators Office/Office of the Governor or that of the Louisiana Applied Oil Spill Research and Development Program, nor does mention of trade names or commercial products constitute endorsement or recommendation for use by the state of Louisiana.
REPORT AVAILABILITY
Questions or requests for this publication should be directed to:
The Louisiana Applied and Educational Oil Spill Research and Development Program (OSRADP)
1995 Deliverables
258 Military Science
Baton Rouge, LA 70803
Telephone Number: (225) 388-3477
Fax Number: (225) 388-0403
or from
The Louisiana Oil Spill Coordinator/Office of the Governor
Natural Resources Building 625 N. 4th St., Rm. 800
Baton Rouge, LA 70802
Telephone Number: (225) 219-5800 Fax Number: (225) 219-5802
CITATION
Nyman, J.A., and W.H. Patrick, Jr. 1996. Effects of oil and chemical responses on fresh marsh function and oil degradation: Response implications. Louisiana Oil Spill Coordinator's Office/Office of the Governor, Louisiana Applied Oil Spill Research and Development Program, Baton Rouge, Louisiana, OSRADP Technical Report Series 95-011.
ACKNOWLEDGEMENTS
Mr. Gerry Canevari (G.P. Canevari and Associates) and Mr. Charles Henry (Institute for Environmental Studies, LSU) provided helpful advice regarding the method of treatment application. Exxon Research and Engineering Company provided the crude oils, the cleaner, and the dispersant. The Miami Corporation, in the person of Mr. Roger Vincent, allowed access to their property collection of soil cores and bulk soil samples, and provided an airboat as well as the services of their biologist, Mr. Billy DeLany. Mr. Mike Windham, (Louisiana Department of Wildlife and Fisheries) suggested locations for soil collection and allowed cores and bulk soils to be collected from the Lake Salvador Wildlife Management Area. Dr. Don Davis (Louisiana Applied Oil Spill Research and Development Program) tolerated much and helped guide this project from pre-proposal to completion.
TABLE OF CONTENTS
- 1.0 Introduction
- 2.0 Methods
- 3.0 Results
- 4.0 Discussion
- 5.0 Conclusions
- 6.0 Response Implications
- 7.0 References
- 8.0 Figures
LIST OF FIGURES
Figure 1. Comparison of the nC-18/phytane biodegradation index for different response treatments on Arabian Crude oil in a Panicum sp. marsh mesocosm before and after incubation for six months
Figure 2. Comparison of the nC-18/phytane biodegradation index for different response treatments on Louisiana Crude oil in a Panicum sp. marsh mesocosm before and after incubation for six months
Figure 3. Comparison of the nC-18/phytane biodegradation index for different response treatments on Arabian Crude oil in a Sagittaria sp. marsh mesocosm before and after incubation for six months
Figure 4. Comparison of the nC-18/phytane biodegradation index for different response treatments on Louisiana Crude oil in a Sagittaria sp. marsh mesocosm before and after incubation for six months
Figure 5. Comparison of the TTAH concentration for different response treatments on Arabian Crude oil in a Panicum sp. marsh mesocosm before and after incubation for six months
Figure 6. Comparison of the TTAH concentration for different response treatments on Louisiana Crude oil in a Panicum sp. marsh mesocosm before and after incubation for six months
Figure 7. Comparison of the TTAH concentration for different response treatments on Arabian Crude oil in a Sagittaria sp. marsh mesocosm before and after incubation for six months
Figure 8. Comparison of the TTAH concentration for different response treatments on Louisiana Crude oil in a Sagittaria sp. marsh mesocosm before and after incubation for six months
Figure 9. Comparison of the TTAH concentration normalized to the C-2 chrysenes for different response treatments on Arabian Crude oil in a Panicum sp. marsh mesocosm before and after incubation for six months
Figure 10. Comparison of the TTAH concentration normalized to the C-2 chrysenes for different response treatments on Louisiana Crude oil in a Panicum sp. marsh mesocosm before and after incubation for six months
Figure 11. Comparison of the TTAH concentration normalized to the C-2 chrysenes for different response treatments on Arabian Crude oil in a Sagittaria sp. marsh mesocosm before and after incubation for six months
Figure 12. Comparison of the TTAH concentration normalized to the C-2 chrysenes for different response treatments on Louisiana Crude oil in a Sagittaria sp. marsh mesocosm before and after incubation for six months
Figure 13. Comparison of the AH histogram profile Arabian Crude oil in a Panicum sp. marsh mesocosm before and after the six month incubation period
Figure 14. Comparison of the AH histogram profile of dispersed Arabian Crude oil in a Panicum sp. marsh mesocosm before and after the six month incubation period
Figure 15. Comparison of the AH histogram profile of Arabian Crude oil mixed with a beach cleaner in a Panicum sp. marsh mesocosm before and after the six month incubation period
Figure 16. Comparison of the AH histogram profile of Arabian Crude oil with fertilizer in a Panicum sp. marsh mesocosm before and after the six month incubation period
Figure 17. Comparison of the AH histogram profile of Louisiana Crude oil in a Panicum sp. marsh mesocosm before and after the six month incubation period
Figure 18. Comparison of the AH histogram profile of dispersed Louisiana Crude oil in a Panicum sp. marsh mesocosm before and after the six month incubation period
Figure 19. Comparison of the AH histogram profile of Louisiana Crude oil mixed with a beach cleaner in a Panicum sp. marsh mesocosm before and after the six month incubation period
Figure 20. Comparison of the AH histogram profile of Louisiana Crude oil with additional fertilizer in a Panicum sp. marsh mesocosm before and after the six month incubation period
Figure 21. Comparison of the AH histogram profile of Arabian Crude oil in a Sagittaria sp. marsh mesocosm before and after the six month incubation period
Figure 22. Comparison of the AH histogram profile of dispersed Arabian Crude oil in a Sagittaria sp. marsh mesocosm before and after the six month incubation period
Figure 23. Comparison of the AH histogram profile of Arabian Crude oil mixed with a beach cleaner in a Sagittaria sp. marsh mesocosm before and after the six month incubation period
Figure 24. Comparison of the AH histogram profile of Arabian Crude oil with additional fertilizer in a Sagittaria sp. marsh mesocosm before and after the six month incubation period
Figure 25. Comparison of the AH histogram profile of Louisiana Crude oil in a Sagittaria sp. marsh mesocosm before and after the six month incubation period
Figure 26. Comparison of the AH histogram profile of dispersed Louisiana Crude oil in a Sagittaria sp. marsh mesocosm before and after the six month incubation period
Figure 27. Comparison of the AH histogram profile of Arabian Crude oil mixed with a beach cleaner in a Sagittaria sp. marsh mesocosm before and after the six month incubation period
Figure 28. Comparison of the AH histogram profile of Louisiana Crude oil with additional fertilizer in a Sagittaria sp. marsh mesocosm before and after the six month incubation period
Figure 29. Average soil respiration rates (g C/hr) in the oil/response scenarios
Figure 30. Soil respiration (g C/hr) among the different oil treatments over time
Figure 31. Percentage of respiration accounted for by methanogenesis in the oil treatments
Figure 32. Soil Eh with depth in microcosms made using soil from Panicum hemitomon marsh and from Sagittaria lancifolia marsh
Figure 33. Soil Eh (mV) averaged over both depths over time among the response treatments
Figure 34. Soil Eh (mV) averaged over both depths over time among the oil treatments
LIST OF TABLES
Table 1. Bulk density and percent water of field soils in the upper 15 centimeters of the marsh and in experimental soils made from bulk samples obtained from the same depth at three Sagittaria falcata marshes and three Panicum hemitomon marshes in coastal Louisiana
Table 2. Grams of oil remaining in microcosms after adding 1.8 grams of crude oil followed by six months incubation as determined gravimetrically via dichloromethane extraction
Abstract
Marshes are important parts of riverine, estuarine, and coastal ecosystems. Because oil is refined, stored, or transported through these areas, some marshes are occasionally oiled. The short-term effects of crude oil on marshes depend primarily on vegetative responses, whereas the long-term effects depend more on the response of the soil microbial community, which in turn controls nutrient remineralization, soil Eh, and oil degradation. Responding to oil spills in Louisiana fresh marshes with physical removal methods is difficult because the peat soils are fragile, and because vegetation may not recover from cutting or trampling. Chemical responses such as cleaners, dispersants, and fertilizers are an option, but deciding whether or not to disperse oil in waterbodies, clean oil on marsh plants, fertilize oiled marsh, or leave the oil for natural degradation requires, among other things, knowledge of how fresh marshes respond to oil and chemical treatments and how oil disappearance is affected by the response strategy. Unfortunately, little is known of the effects of oil spills on microbial community response, particularly in fresh marshes. This is understandable in other areas, but roughly 25% of Louisiana's 4.2 million acres of coastal marshes are fresh; furthermore, most riverine marshes are fresh even outside Louisiana.
We compared soil respiration, soil Eh, and oil disappearance among microcosms containing fresh marsh soils that differed in plant community origin (Panicum hemitomon or Sagittaria lancifolia marshes), petroleum source (Louisiana Crude, Arabian Crude, or no crude oil), and response strategy (a cleaner, a dispersant, nutrient addition, or no response). Six replicates of each of the 24 possible scenarios were incubated for six months. Decreased soil respiration would suggest slowed nutrient remineralization rates and possible long-term negative effects on wetland function. Deceased soil Eh would indicate a more stressful rooting environment for emergent marsh vegetation and would also be valued negatively. Enhanced petroleum degradation should reduce toxic effects on wetland organisms and would be valued positively.
Gas chromatography/mass spectrometer (GC/MS) analyses indicated that the alkane fraction was highly degraded in all treatments by the end of the six month incubation. In addition, the mean concentration of target aromatic hydrocarbons was reduced in all treatments. Selective changes consistent with oil biodegradation were observed and indicate that loss of hydrocarbons resulted from biodegradation rather than from simple migration or evaporation. Although well degraded, the petroleum was less degraded than is typical in field conditions. It is not known if this slower rate of degradation is characteristic of fresh marsh soils, or if it resulted from a lack of rooted vegetation in the microcosms. At the end of the six month incubation period, no significant enhancement in oil biodegradation was observed following any response action when compared to the oil with no response scenario. The only observed differences were between the two oil types: the Louisiana Crude lost a greater percentage of total target aromatic hydrocarbons overall compared to the Arabian Crude.
Gravimetric analyses of dichloromethane extracts indicated that cleaned and dispersed treatments had significantly less oil after six months than no response or fertilizer response scenarios. The difference between the gravimetric analyses and the GC/MS analyses likely resulted from the more general nature of the gravimetric analyses relative to the detailed focus of the GC/MS analyses on the more toxic components of the crude oils.
Soil respiration was not inhibited by crude oil or response action scenarios. Instead, soil respiration was temporally stimulated by Louisiana Crude treated with fertilizer and by all scenarios containing Arabian Crude. Estimates of total C loss throughout the six month incubation were 1,115 g C, 781 g C, and 672 g C from Arabian Crude, Louisiana Crude, and no oil treatments, respectively. The additional C from the microcosms treated with crude oil relative to the unoiled microcosms far exceeds the estimated 1.4 g C added in the form of crude oil. Thus, rather than slowing soil organic matter mineralization and the associated release of nutrients to the emergent plant community, some spill scenarios increased soil organic matter mineralization rates.
The amount of respiration accounted for by methanogenesis was not affected by the response options, but Arabian Crude added to S. lancifolia soil elevated methanogenesis to levels similar to that in P. hemitomon soil. The absolute amount of methane emissions was temporarily stimulated by the dispersant scenarios. These increases in methanogenesis are notable because they did not result in slower soil organic matter mineralization rates as would generally be expected.
Soil Eh was temporally lowered by both crude oils and the three response actions. Of the response treatments, fertilization lowered Eh the most. Both of the crude oils lowered Eh, but the effect occurred sooner in response to Louisiana Crude than in response to Arabian Crude. The effects of lowered soil Eh on emergent marsh vegetation cannot be predicted from this study, but should depend on reductions in the depth of the surface oxidized layer.
The results of these studies can contribute to building fresh marsh response guidelines and also because they highlight the areas where additional information is most needed. Important soil processes in the two dominant fresh marsh plant communities generally responded in a similar manner to all scenarios. If the emergent vegetation also responds similarly, then response coordinators may be able to treat both of these plant communities in the same way. Although gravimetric analyses of oil remaining in the microcosms indicated that cleaning and dispersing crude oil enhanced oil loss, detailed analyses of the more toxic components of the oil with GC/MS indicated no long-term enhancement by the cleaning, dispersing, or fertilization scenarios investigated. Perhaps more importantly, no long-term disadvantages such as slowed soil organic matter mineralization or lower soil Eh were detected in any of the response scenarios investigated, including no response. Thus, the use of the chemical cleaner, dispersant, and fertilization for the purpose of short-term gain, such as reducing vegetation or wildlife mortality, is not prohibited by the long-term soil microbial response. More information is needed however before response strategies in fresh marsh can actually be clarified. Specifically, the following information needs to be determined: (1) the effects of other commonly spilled hydrocarbons such as No. 2 fuel oil (diesel) and Bunker C as well as response scenarios on soil process, (2) the responses of the emergent vegetation to the different scenarios, and (3) the toxicities of the different response scenarios on aquatic organisms that live in fresh marsh. Finally, even though no long-term beneficial effect of fertilization was shown in these studies, it should be worthwhile to explore different fertilizer formulations and application scenarios because other studies demonstrate that the technique holds promise.
1.0 Introduction
1.1 Oil and Marshes
Marshes are important parts of riverine, estuarine, and coastal ecosystems. Because oil is refined, stored, and transported through these areas, some marshes are occasionally oiled. Oil has spilled directly into marshes from pipelines (Mendelssohn et al. 1993) or washed into marshes from adjacent rivers, bayous or lakes (Alexander et al. 1979). Marshes provide fish and wildlife habitat and can improve water quality (Mitsch and Gosselink, 1984:393-414). Thus, there is great public interest in oil spills and response activities that affect marshes.
Previous work indicated that oil can adversely affect marsh vegetation. These adverse effects range from short-term depressions of photosynthesis to near total plant mortality (Baker, 1970; DeLaune et al. 1979; Hershner and Lake, 1980; Webb et al. 1981; Alexander and Webb, 1985; Pezeshki and DeLaune, 1993). Different effects of oil on plants result partly because different oils have a range of toxicity levels and partly because various plant species respond differently to oiling. Adverse effects of oil on vegetation may in turn harm adjacent aquatic habitats where the marsh vegetation provides detritus to food webs. (Mitsch and Gosselink, 1984:173-207). Even if plants are not adversely affected, wildlife that uses oiled marshes may become oiled. Significant wildlife mortality is especially likely along the fringes of rivers, lakes, and bayous. (Alexander et al. 1979).
Little attention has been paid to the effects of oil on the soil microbial community even though microbial processes in marsh soils degrade oil and influence nutrient cycling, plant growth, and ecosystem functions. The microbial community regulates the flow of energy from plants to food webs (Knox, 1986:111-112). Soil conditions, primarily soil Eh, directly control plant growth in marshes (Chalmers, 1982; Good et al. 1982). Changes in soil water chemistry coincide with changes in soil Eh (Feijtel et al. 1988), and soil Eh has been used as an indicator of stress on fresh, brackish, and saline marsh vegetation (DeLaune et al. 1983; Burdick et al. 1989; McKee and Mendelssohn, 1989). The soil microbial community also controls the release of nutrients from marshes to adjacent water.
This continual nutrient release appears to maintain a rapid transfer of energy when demand is high, regardless of primary productivity (Knox, 1986:111-112). The recovery of oiled vegetation may therefore depend partly on biodegradation rates and how oil affects soil conditions. Oil may have long-term effects on wetland functions even after plant growth resumes. Thus, whereas short-term effects of oil spills in marshes are dominated by effects on plants, the long-term functioning of the wetland greatly depends on how oil affects soil microbial processes.
Burns and Teal (1979) found oil in marsh soil seven years after a spill, which indicates
the potential for long-term effects. Some workers found little or no effects of oil
on the soil microbial community (DeLaune et al. 1979; DeLaune et al. 1984), but others have found adverse effects of oil on soil organisms (Alexander
and Schwarz, 1981; Sanders et al. 1980). Few have examined the effects of crude oil on mineralization rates and only
one study used Louisiana Crude. Alexander and Schwarz (1980) found that South Louisiana
Crude and Kuwaiti Crude oils generally stimulated mineralization of 14C-labeled glucose
in four to 20 hour incubations with seawater and estuarine sediment. Oil can have
dramatic effects on Eh when it prevents oxygen from entering the water (DeLaune et al. 1979). Effects vary depending on the oil used.
1.2 Response Strategies
Oil can be collected and skimmed, but this may greatly damage fragile marsh soils and plants (OTA, 1990:30). Some on-scene coordinators have cut and removed all oiled marsh vegetation to prevent contamination of wildlife, but this has led to erosion (Baca et al. 1985). Cutting is therefore not a valuable tool in Louisiana's coastal marshes where marsh loss is already severe (Gagliano et al. 1981). Mendelssohn et al. (1990) found that physical disturbance of vegetation and soil associated with cleanup activities had detrimental and long lasting effects on marsh vegetation. Hoff et al. (1993) found that trampling vegetation caused worse damage to Salicornia/Distichlis marsh than North Slope Crude. Thus, physical responses are generally not advisable in Louisiana fresh marshes.
Chemical responses to oil spills are also available. Dispersants are becoming less toxic and their use may increase (Cunningham et al. 1991), but they are not likely to be deployed directly in marshes because toxicity problems may develop where there is little water to dilute the dispersed oil (OTA, 1990:21). Instead, dispersants should only be used in deep water with good circulation. Even if this condition is met, marshes could still be exposed to dispersant and dispersed oil could be present in adjacent rivers, bayous, or lakes.
A new chemical alternative was recently developed (Fiocco et al. 1991). This is a cleaner rather than a dispersant; it does not disperse oil but allows oil to be washed from rock or vegetation surfaces back into the water where it can be collected. Teas et al. (1993) recently showed that cleaning prevented mortality of oiled red mangroves (Rhizophora mangle) by removing oil that otherwise suffocated roots. DeLaune et al. (1995) showed that cleaning prevented mortality of Spartina alterniflora loisel coated with Bunker C (a common fuel oil). Cleaners might someday be used in marshes fringing rivers, bayous, and lakes in small areas where wildlife use is concentrated.
A third chemical response is the application of nutrients to oiled marshes to help degrade the oil. Nutrients could be applied to spilled oil in waterbodies before the oil reaches the marsh. Nutrients are the only response that could conceivably be applied on a wide scale to marshes that have already been fouled with oil. Thus, chemical responses are more likely than physical responses in fresh marshes. A fourth response to spilled oil may be no action, which has been the case for some rocky shorelines (OTA, 1990:30, Mearns, 1993). This method may work in Louisiana as well because oil evaporates and naturally degrades in wetland soils (DeLaune et al. 1980, Hambrick et al. 1980).
Deciding whether or not to use dispersants, cleaners, or fertilizers depends partly on how marshes respond to oil and chemical treatments. Unfortunately, virtually all previous studies addressed saline marshes. This is understandable in areas where there are few non-saline marshes, but less than 25% of Louisiana's 4.2 million acres of coastal marshes are saline (Chabreck, 1970). There is no information available to evaluate the effects of oil on brackish marshes dominated by wiregrass (Spartina alterniflora) or fresh marshes dominated by paille fine (Panicum hemitomon) or bulltongue (Sagittaria lancifolia). Even the comprehensive text by Bruel (1981) used a classification system that groups all Louisiana marshes together. This was done even though it is widely recognized that the plant species found throughout Louisiana marshes have varying physiologic responses to stress, and that a range of soil processes dominate in the different marsh types (Mitsch and Gosselink, 1984). Chabreck (1970) found that most fresh marshes in Louisiana were dominated by either paille fine or bulltongue. Although both paille fine and bulltongue marshes are classified as fresh, these are distinctly different marshes resulting from different soil and water chemical factors. For instance, Chabreck (1970) noted that bulltongue dominated fresh marshes with greater soil mineral matter content and appeared to tolerate greater exposure to sea water than paille fine.
This knowledge gap may one day affect Louisiana's ability to respond to an oil spill. No information is available to guide response strategy to oil spills that may occur in over 75% of the 4.2 million acres of marshes that pipelines cross in coastal Louisiana. No information is available to guide response strategy to oil spills that may occur in the upper reaches of the Mississippi River Gulf Outlet (MRGO), at the mouth of the Mississippi River, at the mouth of the Atchafalaya River, in Wax Lake Outlet, or in 90% of the Gulf Intracoastal Waterway (GIWW).
1.3 Research Needs
A need therefore exists for information regarding oil biodegradation, oil toxicity, and ecological effects of chemical responses in Louisiana fresh marshes. Important questions include: how sensitive are fresh marshes to oil? How are soil processes in fresh marshes affected by dispersants and cleaners? Is the biodegradation rate increased, decreased, or unaffected by chemical responses? How are other important soil processes that control plant growth and ecosystem function affected by oil and chemical responses? Do the most common crude oils in Louisiana affect fresh marsh similarly of differently?
To answer these questions, we compared soil respiration, soil Eh, and oil disappearance among microcosms containing fresh marsh soils that differed in plant community origin (Panicum hemitomon or Sagittaria lancifolia marshes), petroleum source (Louisiana Crude or Arabian Crude), and response strategy (a cleaner, a dispersant, nutrient addition, or no response). Incubations lasted six months. Decreases in CO2 or CH4 emission rates were causes of concern with regard to soil respiration and nutrient cycling. This information was used to help determine the following:
- Which response strategy (no action, application of cleaners, application of dispersants, application of fertilizer) resulted in the greatest evaporation and biodegradation of crude oil in fresh marshes.
- Which response strategy (no action, application of cleaners, application of dispersants) resulted in the least adverse effect on soil respiration and soil Eh in fresh marshes.
- If response strategies should be the same or different in the two most common fresh marshes.
- If response strategies should be the same or different for the two most common crude oils transported in Louisiana.
2.0 Methods
The study created 144 fresh marsh microcosms by placing Louisiana fresh marsh soils in flasks that were subjected to one of three oiling scenarios: Louisiana Crude, Arabian Crude, or no oil. Each flask was also subjected to one of four response scenarios: no response, dispersed oil entering marsh, cleaned oil entering marsh, or addition of fertilizer to the marsh. There were thus 12 treatment combinations. Soil respiration and soil Eh were monitored following treatment implementation. The disappearance of oil was compared among the treatments gravimetrically as well as with gas chromatography/mass spectrometer (GC/MS) analyses. Detailed descriptions follow.
Soil cores and bulk samples were collected from three areas in coastal Louisiana at approximately one month intervals during August and September 1994. Study areas were as follows: near Lake Misere in Cameron Parish, near Lake Salvador in St. Charles Parish, and adjacent to the Tchefuncte River in St. Tammany Parish. Within each area, two 15 cm diameter cores were collected from a Panicum hemitomon marsh, and two from a Sagittaria lancifolia marsh. These cores were returned to the lab and used to characterize organic matter content, moisture content, and soil bulk density within the upper 15 cm of field soils. Soil Eh was also measured in the field with duplicate, Pt electrodes at the surface and at 2.5 cm below the surface depths. Eh was inadvertently measured at three and five cm below the surface at one site (Lake Misere).
Within each area, separate bulk samples were collected from Sagittaria lancifolia marsh and Panicum hemitomon marsh. Bulk soil was collected from the upper 15 centimeters, placed in covered, plastic tubs, and returned to the lab. Tubs were wrapped with a space blanket to prevent temperature increases during transport back to the lab. Each bulk sample was homogenized; large tubers of S. lancifolia were removed. Even though no additional water was added to the bulk soil samples, all soils converted from firm peats to fluid pastes following destruction of the living root network. Three hundred ml of soil were placed in clean, preweighed and numbered 500 ml Erlenmeyer flasks, which were reweighed to determine the mass of soil added. Five 300 ml samples were sacrificed and dried to determine moisture content, oven dried weight, and bulk density. Soils were allowed to rest at least seven days before treatments were added; during this time the soils formed floating, saturated mats in the flasks, a reaction that is typical of Louisiana fresh marshes (Sasser, 1994). Forty-eight flasks were prepared for incubation from each area, making a total of 144 flasks. An additional 48 flasks were prepared with soil from the Lake Salvador area for initial GC/MS analyses.
Three subsamples of each homogenized soil sample were placed in reweighed containers, weighed wet, dried at 100 C, and reweighed to determine bulk density. Organic matter content of the samples was determined on a subsample of the dried samples via combustion at 400 °C for 12 hours (Davies, 1974).
Louisiana Crude (100 ml), Arabian Crude (100 ml), dispersed oil (100 ml oil and 20 ml COREXIT 9550), and cleaned oil (100 ml oil and 20 ml COREXIT 9580) were weathered in 1000 ml beakers containing 100 ml of deionized water. Each beaker was continuously stirred under a fume hood overnight (16 hours). After weathering, oil fractions were separated from water fractions with a separatory funnel, and each fraction was stored in an amber glass jar until it was applied to the microcosms.
Two ml of oil fraction (1.8 g) and six ml (6 g) of the water fraction were added to the appropriate microcosm. This oil to water ratio was used to maintain a 1:3 ratio during weathering. This volume of oil was added to microcosms because preliminary trials indicated that it produced approximately 75% coverage of the surface area in similar flasks containing similar amounts of soil. The fertilizer solution was a 0.09 M (NH4)2HPO4 solution prepared from stock chemicals; 10 ml were added to appropriate flasks. This application rate was equivalent to 4.1 g N/m2 and 4.5 g P/m2. For microcosms receiving cleaner or dispersant but no oil, 0.4 ml of chemical in 10 ml of deionized water were added to each flask. Our oil/substrate ratio (1:150) was greater than that used by Foght et al. (1987, 1:1,000) in their study of freshwater (1987, 1:1,000) dispersants on lake sediments. Our oil/dispersant and oil/cleaner ratio (1:5) was greater than that used by Foght et al. (1987, 1:10).
Replicate flasks were prepared with soil from each marsh area, making 144 microcosms. An additional set of 48 microcosms were prepared and sacrificed immediately after treatment implementation. These flasks were frozen and delivered to the Institute for Environmental Studies where they were held until the final samples were ready for analysis.
The soil in the microcosms was kept shallowly flooded. It was not possible to flood them with more than a centimeter or two of water because the soils formed floating mats in the microcosms. Floating marshes are common in Louisiana (Sasser, 1994) and marshes at two of our three sample areas were buoyant. Our microcosms behaved similarly to those floating marshes, which rise and fall with water levels but remain saturated and often have a centimeter or two of water on their surfaces. Deionized water was added to the flasks when the soil surface dropped below the original soil level; this allowed the soil surface to become occasionally exposed to air but never dry.
Carbon dioxide and CH4 emissions were measured at roughly one, three, seven, 14, 28, 84, and 180 days after treatments were applied. Flasks were sealed with a rubber stopper equipped with a rubber septum. Air samples (0.5 ml) were collected with a gas syringe and immediately injected into gas chromatography. CO2 and CH4 were detected with a flame ionization detector. Carbon dioxide was converted to methane with a methanizer heated to 300 C. Commercially available reference gases and peak analyses software (EZ Chrom, Chromatography Data System) were used to calculate concentrations of CO2 and CH4 in samples. A second air sample was collected at least 90 minutes after the first sample was collected and the change concentration between the two measurements was calculated.
Redox potential was measured in microcosms with duplicate bright platinum electrodes (Faulkner et al. 1989) at the soil surface and at 2.5 cm below the soil surface throughout the incubation. Eh was measured before treatment implementation and up to 112 days after treatment implementation.
After six months of incubation, microcosms from the Lake Salvador site were frozen for GC/MS analyses and delivered to the Institute for Environmental Studies, LSU for extraction and analysis. Petroleum was extracted from the remaining 96 microcosms with dichloromethane and determined gravimetrically. Gravimetric analyses were analyzed as a 2*3*4 ANOVA: vegetation type by oiling-scenario by response-scenario with blocking on site.
Flasks at the Institute for Environmental Studies were frozen at either time zero (day zero) or at the completion of six months of incubation. Freezing allowed the before and after flasks to be analyzed together and thereby minimized technique variability. Each flask was broken in the frozen state and split into a top and bottom half. The respective halves were placed into precleaned jars with Teflon lined caps and mixed (homogenized). A subsample (10 g wet sediment) of each was removed and extracted with nanograde purity dichloromethane. At the time of extraction, internal surrogate standards were added to assess recovery. After extraction the sample extract was reduced to a final volume of 1 ml. Each extract was passed through a normal phase chromatography column composed of conditioned silica-gel and alumina to remove polar biogenic interferences extracted from the freshwater mesocosms with the oil constituents. The final extraction volume was adjusted between 0.5 and 16 ml (concentration dependent) before GC/MS analysis.
Specific alkane ratios and target aromatic hydrocarbon end-points were monitored with GC/MS analyses to assess efficacy. Specific analytical procedures are documented separately (Henry and Overton, 1993; Roques and et al. 1994). The GC/MS results provide specific compositional changes in a wide range of aromatic hydrocarbons. Aromatic hydrocarbons are the most significant endpoint from a biological perspective because of their toxicity and resistance to microbial degradation.
Soil Eh data were measured as a split plot (depth) with repeated measures (time) using Proc GLM of SAS; 2,301 observations were analyzed. Eh data did not require transformation prior to analyses to achieve normality. Effects with an P < 0.05 were considered statistically significant; insignificant interaction terms were pooled into the appropriate error term to improve error estimate.
Soil respiration was estimated from rate of CO2 and CH4 emissions and expressed in terms of total C emission rates. These data were analyzed as 2x3X4 factorial (plant type by oil treatment by response) with repeated measures over time; 968 observations were analyzed. Log transformations were used to make the data normal. Effects with a P < 0.05 were considered statistically significant; insignificant interaction terms were pooled into the appropriate error term to improve error estimate.
The percentage of soil respiration accounted for by methanogenesis was similarly analyzed. Zero estimates were set to 0.0001 and data were log transformed to improve normality; 962 observations were analyzed.
The absolute amounts of CO2 and CH4 were similarly analyzed. Carbon dioxide data were transformed with the (log +1); CH4 data were log transformed to improve normality; 974 observation of CO2 and CH4 were analyzed.
3.0 Results
3.1 Soil Characteristics
Organic matter content in the microcosm soils ranged from 41% to 90% and averaged 52% in the S. falcata soil and 66% in the P. hemitomon soils. Bulk density was five times greater in the microcosm soils than in the field soils (Table 1). Moisture content was extremely variable but was generally greater for field soils of S. falcata marsh, but greater for microcosm soils of P. hemitomon marsh (Table 1). These differences likely resulted from a large degassing of the field soils and below ground root material during soil preparation.
Table 1. Bulk density and percent water of field soils in the upper 15 cm of the marsh and in experimental soils made from bulk samples obtained from the same depth at three Sagittaria falcata marshes and three Panicum hemitomon marshes in coastal Louisiana.
| Source Marsh | Bulk Density (g/cm3) | Percent Water | ||
|---|---|---|---|---|
| field | microcosm | field | microcosm | |
| S. falcata marshes | ||||
| Lake Misere | 0.014 | 0.059 | 126 | 143 |
| Lake Salvador | 0.016 | 0.128 | 112 | 82 |
| Tchefuncte River | 0.032 | 0.126 | 70 | 82 |
| Average of S.falcata | 0.021 | 0.106 | 103 | 102 |
| P hemitomon marshes | ||||
| Lake Misere | 0.014 | 0.074 | 83 | 138 |
| Lake Salvador | 0.012 | 0.042 | 142 | 236 |
| Tchefuncte River | 0.021 | 0.152 | 85 | 69 |
| Average of P. hemitomon | 0.016 | 0.890 | 103 | 148 |
3.2 Oil Remaining
The amount of oil remaining in the microcosms after incubation as determined by gravimetric analyses differed among the scenarios (F = 2.24, 6 and 82 df, P = 0.0471). The amount of oil remaining differed significantly from 0 g only in oiled microcosms (Table 2). There was no difference between oiled microcosms receiving fertilizer and oiled microcosms receiving no response, whereas oiled microcosms treated with cleaner or dispersant had significantly less oil than the oiled microcosms receiving no response (Table 2). There was no difference in the amount of oil remaining between the oiled flasks treated with cleaners and those treated with dispersant (Table 2).
Table 2. Grams of oil remaining in microcosms after adding 1.8 grams of crude oil followed by six months incubation as determined gravimetrically via dichloromethane extraction. Each estimate is the least square mean of eight samples; least square standard errors are in parenthesis.
| Oil Treatment | Response Treatment | |||
|---|---|---|---|---|
| no response | cleaner | dispersant | fertilizer | |
| no oil | <0.001 (0.065) | 0.003 (0.065) | 0.046 (0.065) | 0.006 (0.065) |
| Arabian Crude | 0.687 (0.065) | 0.414 (0.065) | 0.480 (0.065) | 0.608 (0.065) |
| Louisiana Crude | 0.672 (0.065) | 0.360 (0.065) | 0.330 (0.065) | 0.613 (0.065) |
3.3 Oil Characterization
Alkane degradation. Overall, all treatments showed highly degraded alkane patterns. In all treatments, all (or most) of the normal alkanes were completely degraded. Only partially degraded isoprenoid compounds remained at the end of six months. Figures 1 through 4 are histogram plots comparing changes in the nC-18/phytane index as well as the four different response treatments at time zero and at the end of the six month incubation period for both marsh and oil types. The nC-18/phytane index is the ratio of the normal hydrocarbon C-18 to the isoprenoid phytane. Bacteria preferentially degrade the normal hydrocarbon at a faster rate than the phytane, therefore changes in this ratio indicate the extent of microbial degradation. The index is useful in reducing sample variability since it is concentration independent, but the index is only useful until the normal hydrocarbon becomes highly reduced. Index values between zero and 0.5 are essentially the same and reflect a highly degraded oil. In all treatments, the extent of alkane degradation was essentially the same.
Aromatic hydrocarbon degradation. Figures 5 through 8 show histogram plots of the
changes in TTAH for all treatments. A relatively high degree of patchiness was observed
even in the time samples. In an effort to overcome this variance, the TTAH values
were normalized to the very biodegradative resistant group of homologues, the C-2
chrysenes. Figures 9 through 12 show histogram plots of the TTAH normalized to C-2
chrysene. The results show that all of the treatments resulted in an approximate 50%
reduction in the TTAH. The results don't highlight any enhanced degree of aromatic
degradation specific with treatment type. Figures 13 through 28 show histogram comparisons
of the target aromatic hydrocarbons at time zero and after the six month incubation
period for each oil, treatment, and marsh type combination. Overall, the changes in
the AH profile were consistent in each treatment. Each treatment reached the same
extent of oil biodegradation at the end of a six month incubation. The only observed
difference were between the two oil types: the Louisiana Crude lost a greater percentage
of TTAH overall when compared to the Arabian Crude. The loss was primarily naphthalene
hydrocarbons. The greater degradation of TTAHs in Louisiana Crude might result partly
from different amounts of 3-ring sulfur hetrocyclic compounds and 2-ring naphthalene
compounds in the two crude oils. Arabian Crude oils contain more 3-ring sulfur hetrocyclic
compounds, and Louisiana Crude oils contain 2-ring naphthalene compounds. Data indicated
that the 3-ring sulfur hetrocyclic compounds were more resistant to degradation than
the 2-ring naphthalene compounds.
3.4 Soil Respiration
Soil respiration varied among the 12 oil and response combinations (F = 2.37, 6 and 124 df, P = 0.0338). Post-ANOVA comparisons with Least Square Means indicated that no treatment combination had slower soil respiration than untreated microcosms (Figure 29). However, soil respiration was faster in treatments containing Arabian Crude and the Louisiana Crude treated with fertilizer than untreated microcosms and all other treatment combinations (Figure 29).
Respiration was 1.3 times faster in P. hemitomon soils than in S. lancifolia (F = 10.37, 1 and 118 df, P =0.0017), but no difference was detected in the way the marsh types responded to the oil treatments (F = 0.38, 2 and 118 df, P = 0.6840) or response treatments (F = 0.40, 3 and 118 df, P = 0.7536).
Significant differences were detected in soil respiration among the oil treatments over time (F = 13.28, 12 and 800 df, P = 0.0 01). Respiration was initially similar among the three treatments, but differences appeared after adding crude oil (Figure 30). Respiration increased by day three in microcosms treated with Arabian Crude, but not until day 14 in microcosms treated with Louisiana Crude (Figure 30). Respiration in microcosms treated with crude oil remained elevated until at least three months after addition of crude oil (Figure 30). There were no differences in respiration among the oiled and unoiled microcosms six months after crude oils were added (Figure 30).
Estimates of total C loss throughout the six month incubation were 1,115 g C, 781 g C, and 672 g C respectively from Arabian Crude, Louisiana Crude, and no oil treatments. The additional 443 g C from the microcosms treated with Arabian Crude and the additional 109 g C from the microcosms treated with Louisiana Crude relative to the unoiled microcosms far exceed the estimated 1.4 g C added in the form of crude oil (1.8 g crude oil, 80% C).
Averaged over all sampling dates, methanogenic carbon accounted for 11% of soil respiration.
The percent of respiration accounted for by methanogenic carbon differed among the
oil treatments over time (F = 2.29, 12 and 624 df, P = 00074) and among the response
treatments over time (F = 1.94, 18 and 624 df, P = 0.0110), but no clear trends were
evident (Figure 31). The percent of respiration accounted for by methanogenesis also
varied among the oil treatment/marsh type combinations (F = 3.75, 2 and 72 df, P =
0.0283). Post-ANOVA comparisons indicated that methane accounted for a greater percentage
of C-emissions in P. hemitomon soil, than in all S. lancifolia soils except those treated with Arabian Crude.
3.5 Soil Eh
Soil Eh was roughly 30 mV higher (F = 6.43, 1 and 106 df, P = 0.0127) in S. lancifolia soil than in P. hemitomon soil, and roughly 50 mV higher at the surface than 2.5 cm below the surface (F = 117.58, 1 and 84 df, P = 0.0001) (Figure 32).
Eh varied over time among the oil and response treatments (F = 2.25, 54 and 1,788 df, P = 0.0001). Interpreting this interaction was difficult because Eh varied less than 50 mV among treatments, but it was clear that Eh was lower in all treatments, including controls, in the beginning of the incubations than at the end. Eh was near 350 when the treatments were added and near 500 when the incubations ended. No differences in Eh were evident among the treatments by the end of the incubations, but Eh was higher in the controls than in treated microcosms initially. Of the response treatments, fertilization lowered Eh the most (Figure 33). Both of the crude oils lowered Eh, but the effect occurred sooner in response to Louisiana Crude than in response to Arabian Crude (Figure 34).
4.0 Discussion
The slow rate of aromatic hydrocarbon degradation relative to alkane degradation observed in this study resembles observations from field studies. Results from studies of spilled oil in an intertidal marsh in Prince William Sound, Alaska, five years after being oiled showed similar results to those reported in this study (Henry, unpublished data). The marsh sediment samples from Alaska, like the sediments in this study, exhibited highly degraded alkane profiles, but very little microbial degradation of aromatic hydrocarbons. Similarly, residual hydrocarbons from oil spilled in an intertidal marsh at Buzzards Bay, Massachusetts by the barge Florida incident in 1969 could still be detected in 1990 (Teal and et al. 1992). The availability of easier to degrade carbon substrates may be the controlling factor.
The residual toxicity associated with the aromatic hydrocarbons and their persistence emphasizes the need for continued research to develop treatment technologies that will enhance aromatic hydrocarbon degradation in sensitive habitats such as the marsh types in this study.
Fertilizers' inability to speed degradation is surprising in light of both the Alaskan experience and unpublished work in Texas. It is important to remember that the positive effect of fertilizers in Alaska was temporary and not apparent after six weeks.
Gravimetric analyses of dichloromethane extracts indicated that cleaned and dispersed treatments had significantly less oil after six months than no response or fertilizer response scenarios. The difference between the gravimetric analyses and the GC/MS analyses likely resulted from the more general nature of the gravimetric analyses compared to the detailed focus of the GC/MS analyses on the more toxic components of the crude oils.
Soil respiration was not inhibited by crude oil or response action scenarios. Instead, soil respiration was temporally stimulated by Louisiana Crude treated with fertilizer and by all scenarios containing Arabian Crude. Estimates of total C loss throughout the six month incubation were 1,115 g C, 781 g C, and 672 g C from Arabian Crude, Louisiana Crude, and no oil treatments, respectively. The additional C from the microcosms treated with crude oil relative to the unoiled microcosms far exceeds the estimated 1.4 g C added in the form of crude oil. Thus, rather than slowing soil organic matter mineralization and the associated release of nutrients to the emergent plant community, some spill scenarios increased soil organic matter mineralization rates.
No previous study has documented stimulation of soil organic matter mineralization by crude oils. However, Alexander and Schwarz (1980) noted that South Louisiana Crude and Kuwaiti Crude oils stimulated glucose mineralization.
The fertilized flasks containing oil had the fastest CO2 emission rates even though they retained as much oil as the untreated flasks. This did not seem to be an effect of the fertilizer alone; it was only apparent when the fertilizer and oil occurred together. The increase in soil organic matter mineralization that we observed might be related to co-oxidation. Co-oxidation, or co-metabolism, is the oxidation of a compound that an organism cannot use as a source of energy or nutrients. Co-oxidation apparently provides no benefit to the organism but has been considered important in crude oil degradation because many hydrocarbons apparently cannot be used as an energy or nutrient source by any organism. The reverse situation, co-oxidation of soil organic matter by organisms metabolizing hydrocarbons, might explain the increase in soil organic matter mineralization observed in our study, but this situation has never been observed before. Another more likely explanation is that the crude oils contained micro-nutrients limiting microbial growth in the marsh soils.
In addition to respiration, CO2 varied among the oiling scenarios over time. The Arabian Crude treatments showed an early flush of CO2 at two weeks; the Louisiana Crude treatments showed a flush at about four weeks. There was no flush in the no-response treatments.
Methane emissions varied among the oiling scenarios over time. Methane emissions generally did not differ among the oiling scenarios, but on three occasions one of the treatments showed faster methane emissions than others.
Methane emissions also varied among the response scenarios over time. Methane emissions were greater in the dispersant scenarios at the outset of the incubations, but no differences were apparent by the end of the incubations. The increase in methane emissions is cause for concern because it strongly suggests reducing conditions in the soil that inhibit plant growth.
The amount of respiration accounted for by methanogenesis was not affected by the response options, but Arabian Crude added to S. lancifolia soil elevated methanogenesis to levels similar to that in P. hemitomon soil. The absolute amount of methane emissions was temporarily stimulated by the dispersant scenarios. These increases in methanogenesis are notable because they did not result in slower soil organic matter mineralization rates as would generally be expected.
Soil Eh was temporally lowered by both crude oils and the three response actions. Of the response treatments, fertilization lowered Eh the most. Both of the crude oils lowered Eh, but the effect occurred sooner in response to Louisiana Crude than in response to Arabian Crude. The effects of lowered soil Eh on emergent marsh vegetation cannot be predicted from this study, but should depend on how much the depth of the surface oxidized layer is reduced.
5.0 Conclusions
- Gravimetric analyses indicated that the cleaner and dispersant enhanced long-term total hydrocarbon losses. But more detailed analyses with GC/MS analyses indicated no long-term beneficial effects on the more toxic, harder to degrade components of Louisiana Crude and Arabian Crude.
- There was no indication of decreased soil respiration in response to Louisiana and Arabian Crude; there was no indication of reduced nutrient mineralization rates. Instead, crude oils caused temporary increases in soil respiration, which suggests that crude oils stimulated the soil microbial community.
- There was no indication of decreased soil respiration in response to a cleaner, a dispersant, or a fertilizer. Instead, the response actions studied caused temporary increases in soil respiration, which suggests that they also stimulated the soil microbial community.
- Based on important soil processes and oil disappearance, different response scenarios are not needed for the two different plant communities that dominate Louisiana fresh marsh. However, the responses of the emergent plant communities must also be considered before new response actions are formulated.
- All three response options temporarily lowered soil Eh. This confirms the conclusion that response options stimulated the soil microbial community. Of the responses, fertilization lowered soil Eh the most. The effects that lowered soil Eh have on emergent marsh vegetation cannot be predicted but will depend on reductions in the depth of the surface oxidized layer. These effects should be determined before new response actions are formulated since decreased soil Eh is an indicator of plant stress.
- Both crude oils temporarily lowered soil Eh. This confirms the conclusion that crude oils stimulated the soil microbial community. The effects that lowered soil Eh has on emergent marsh vegetation cannot be predicted but will depend on reductions in the depth of the surface oxidized layer.
6.0 Response Implications
The results of these studies can contribute to building fresh marsh response guidelines because of the information they produced, and also because they highlight the areas where additional information is most needed. Important soil processes in the two dominant fresh marsh plant communities generally responded similarly to all scenarios. If the emergent vegetation also responds similarly, then response coordinators may be able to treat both of these plant communities in the same way. Although gravimetric analyses of oil remaining in the microcosms indicated that cleaning and dispersing crude oil enhanced oil loss, detailed analyses of the more toxic components of the oil with GC/MS indicated no long-term enhancement by the cleaning, dispersing, or fertilization scenarios investigated. Perhaps more importantly, no long-term disadvantages such as slowed soil organic matter mineralization or lower soil Eh were detected in any of the response scenarios investigated, including no response. Thus, the use of chemical cleaner, dispersant, and fertilization for the purpose of short-term gain, such as reducing vegetation or wildlife mortality, is not prohibited by the long-term soil microbial response. More information is needed however before response strategies in fresh marsh can actually be clarified. Specifically, the following needs to be determined:
- the effects of other commonly spilled hydrocarbons such as No. 2 fuel oil (diesel) and Bunker C as well as response scenarios on soil process,
- the responses of the emergent vegetation to the different scenarios, and
- the toxicities of the different response scenarios on aquatic organisms that live in fresh marsh.
Finally, even though no long-term beneficial effect of fertilization was shown in these studies, it would be worthwhile to explore different fertilizer formulations and application scenarios because other studies demonstrate that the technique holds promise.
7.0 References
- Alexander, S.K., and J.R. Schwarz. 1980. Short-term effects of South Louisiana and Kuwait Crude oils on glucose utilization by marine bacterial populations. Applied and Environmental Microbiology. 40:341-345.
- Alexander, S.K., and J.W. Webb, Jr. 1985. Oil in the salt marsh: What have we learned? In: C.F. Bryan et al. (eds.), Proceedings of the Forth Coastal Marsh and Estuary Management Symposium, Louisiana State University Printing Office, Baton Rouge, Louisiana. pp. 49-62.
- Alexander, M.M., P. Longabucco, and D.M. Phillips. 1979. The impact of oil on marsh communities in the St. Lawrence River. Proceedings of the 1979 Oil Spill Conference. 1979:333-340.
- Baca, B.J., C.D. Getter, and J. Lindstedt-Siva. 1985. Freshwater oil spill considerations: Protection and cleanup. Proceedings of the 1985 Oil Spill Conference. pp. 385-390.
- Baker, J.M. 1970. The effects of oil on plants. Environmental Pollution. 1:27-44.
- Breuel, A. 1981. Oil spill cleanup and protection techniques for shorelines and marshlands. Pollution Technology Review No. 78. Noyes Data Corp., Park Ridge New Jersey.
- Burdick, D.M., I.A. Mendelssohn, and K.L. McKee. 1989. Live standing crop and metabolism of the marsh grass Spartina patens as related to edaphic factors in a brackish, mixed marsh community in Louisiana. Estuaries. 12:195-204.
- Burns, K.A., and J.M. Teal. 1979. The West Falmouth oil spill: Hydrocarbons in the salt marsh ecosystem. Estuarine and Coastal Marine Science. 8:349-360.
- Chabreck, R.H. 1970. Marsh zones and vegetative types of the Louisiana coastal marshes. Ph.D. Dissertation. Louisiana State University, Baton Rouge Louisiana. 112pp.
- Chalmers, A.G. 1982. Soil dynamics and the productivity of Spartina alterniflora. In: V.S. Kennedy (ed.), Estuarine Comparisons. Academic Press: New York. pp. 389-393.
- Cunningham, J.M., K.A. Sahatjian, C. Meyers, G. Yoshioka, and J.M. Jordan. 1991. Use of dispersants in the United States: Perception or reality? Proceedings of the 1991 Oil Spill Conference. pp. 389-393.
- de la Cruz, A.A., and C.T. Hackney. 1989. Temporal and spatial patterns of redox potential (Eh) in three tidal marsh communities. Wetlands. 9(2):181-190.
- DeLaune, R.D., W.H. Patrick, Jr., and R.J. Buresh. 1979. Effect of crude oil on a Louisiana Spartina alterniflora salt marsh. Environmental Pollution. 1979:21-31.
- DeLaune, R.D., G.A. Hambrick, and W.H. Patrick, Jr. 1980. Degradation of hydrocarbons in oxidized and reduced sediments. Marine Pollution Bulletin. 11:103-106.
- DeLaune, R.D., C.J. Smith, and W.H. Patrick, Jr. 1983. Relationship of marsh elevation, redox potential, and sulfide to Spartina alterniflora productivity. Soil Sci. Soc. Am. J. 47:930-935.
- DeLaune, R.D., C.J. Smith, W.H. Patrick, Jr., J.W. Fleeger, and M.D. Tolley. 1984. Effect of oil on salt marsh biota: Methods for restoration. Environmental Pollution (A). 36:207-227.
- Faulkner, S.P., W.H. Patrick, Jr., and R.P. Gambrell. 1989. Field techniques for measuring wetland soil parameters. Soil Sci. Soc. Am. J. 53:883-890.
- Feijtel, T.S., R.D. DeLaune, and W.H. Patrick, Jr. 1988. Seasonal porewater dynamics in marshes of Barataria Basin, Louisiana. Soil Sci. Soc. Am. J. 52:59-67.
- Fiocco, R.J., G.P. Canevari, J.B. Wilkinson, H.O. Jahns, J. Bock, M. Robbins, and R.K. Markarian. 1991. Development of COREXIT 9580: A chemical beach cleaner. Proc. 1991. Oil Spill Conf. American Petroleum Institute: Washington D.C. pp. 395-400.
- Fleeger, J.W., and G.T. Chandler. 1983. Meiofauna responses to an experimental oil spill in a Louisiana salt marsh. Marine Ecology Progress Series. 11:257-264.
- Foght, J.M., N.J. Fairbairn, and D.W.S. Westlanke. 1987. Effect of oil dispersants on microbially-mediated processes in freshwater systems. In: J.H. Vandermeulen and S.E. Hrudey (eds.), Oil in Freshwater: Chemistry, Biology, Countermeasure Technology. Proceedings of the Symposium of Oil Pollution in Freshwater, Edmonton, Alberta, Canada. Pergamon Press.
- Gagliano, S.W., K.J. Meyer-Arendt, and K.M. Wicker. 1981. Land loss in the Mississippi River Deltaic Plain. Trans. Gulf Coast Assoc. Geol. Soc. 31:295-300
- Good, R.E., N.F. Good, and B.R. Frasco. 1982. A review of primary production and decomposition dynamics of the belowground marsh component. In: V.S. Kennedy (ed.), Estuarine Comparisons. Academic Press. pp. 139-157.
- Hambrick, G.A., R.D. DeLaune, and W.H. Patrick, Jr. 1980. Effect of estuarine sediment pH and oxidation-reduction potential on microbial hydrocarbon degradation. Applied and Environmental Microbiology. 40(2):365-369.
- Henry, C.B., and E.B. Overton. 1993. Source-fingerprinting and compound specific quantitative analysis of oil contaminated soils and sediments. Institute for Environmental Studies Technical Report: IES93-01; unpublished manuscript.
- Hershner, C., and J. Lake. 1980. Effects of chronic oil pollution on a salt-marsh grass community. Marine Biology. 56:163-173.
- Hoff, R.Z., g.Shigenaka, and C.B. Henry. 1993. Salt marsh recovery from a crude oil spill: Vegetation, oil weathering, and response. 1993 Oil Spill Conference. 1993:307-311.
- Johnson, T.L., and R.A. Pastorok. 1985. Oil spill cleanup! Options for minimizing adverse ecological impact. American Petroleum Institute. Publication No. 4435.
- Knox, G.A. 1986. Estuarine ecosystems: A systems approach. Volume II. CRC Press, Inc. : Boca Raton FL.
- La DNR. 1991. Louisiana Energy Statistics, 1909-1989. Center for Energy Studies, 1 E. Fraternity Circle, LSU. Baton Rouge LA.
- McCree, K.J. 1986. Measuring the whole-plant daily carbon balance. Photosynthetica. 10:82-93.
- McKee, K.L., and I.A. Mendelssohn. 1989. Response of a freshwater marsh plant community to increased salinity and water level. Aquat. Bot. 34:301-316.
- Mearns, A.J. 1993. Recovery of shoreline ecosystems following the Exxon Valdez oil spill and subsequent treatment. In: O.T. Magoon, (ed.), Coastal Zone '93 Volume I. American Society of Civil Engineers, New York. pp. 466-479.
- Mendelssohn, I.A., M.W. Hester, and C. Sasser. 1990. The effect of a Louisiana Crude oil discharge from a pipeline break on the vegetation of a Southeast Louisiana brackish marsh. Oil and Chemical Pollution. 7:1-15.
- Mendelssohn, I.A., M.W. Hester, and J.M. Hill. 1993. Assessing the recovery of coastal wetlands from oil spills. Proceedings 1993 International Oil Spill Conference. pp. 141-145.
- Mitsch, W.J., and J.G. Gosselink. 1984. Wetlands. Van Nostrand Reinhold Co: New York.
- OTA. 1990. Coping with an oiled sea. U.S. Congress, Office of Technology Assessment. OTA-BP-O-63. U.S. Government Printing Office, Washington D.C.
- Pezeshki, R.S., and R.D. DeLaune. 1993. Effect of crude oil on gas exchange functions of Juncus romerianus and Spartina alterniflora. Water, Air, and Soil Pollution. 68:461-468.
- Roques, D.E., et al. 1994. Using gas chromatography mass spectrometry fingerprint analyses to document process and progress of oil biodegradation. Journal of Environmental Quality. 23(4): 851-855.
- Sanders, H.L., J.F. Grassle, G.R. Hampson, L.S. Morse, S. Garner-Price, and C.C. Jones. 1980. Anatomy of an oil spill: Long-term effects from the grounding of the barge Florida off West Falmouth, Massachusetts. Journal of Marine Research. 38(2):265-380.
- Smith, C.J., R.D. DeLaune, W.H. Patrick, Jr., and J.W. Fleeger. 1984. Impact of dispersed and undispersed oil entering a Gulf Coast salt marsh. Environmental Toxicology and Chemistry. 3:609-616.
- Steele, R.G.D., and J.H. Torrie. 1980. Principles and procedures of statistics: A biometrical approach. Second Edition. McGraw-Hill, Inc. New York. 633pp.
- Teal, J.M., et al. 1992. The West Falmouth oil spill after 20 years: Fate of fuel oil compounds and effects on animals. Marine Pollution Bulletin. 24(12): 607-614.
- Teas, H.J., R.R. Lessard, G.P. Canevari, C.D. Brown, and R. Glenn. 1993. Saving oiled mangroves using a new non-dispersing shoreline cleaner. Proc. 1993. International Oil Spill Conf. American Petroleum Institute: Washington D.C.
- US DOE. 1993. Petroleum Supply Annual 1992. Energy Information Administration, Office of Oil and Gas, U.S. Dept. of Energy, Washington D.C.
- Venosa, A.D., M. Kadkhodayan, D.W. King, B.A. Wrenn, J.R. Haines, T. Herrington, K. Strohmeier, M.T. Suidan. 1993. Testing the efficacy of oil spill bioremediation products. Proc. 1993 International Oil Spill Conf. 1993:487-493.
- Webb, J.W., G.T. Tanner, and B.H. Koerth. 1981. Oil spill effects on smooth cordgrass in Galveston Bay, Texas.
8.0 Figures
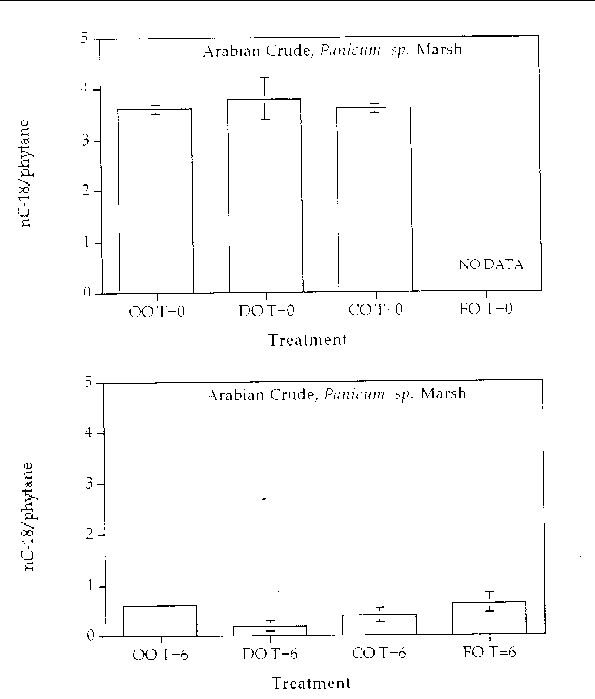 Figure 1. Comparison of the nC-18/phytane biodegradation index for different response treatments
on Arabian Crude oil in a Panicum sp. marsh mesocosm before (top) and after incubation (bottom) for six months. For all
treatments n=2. Key: OO-Oil Only, DO-Dispersed Oil, CO-Cleaned Oil, and FO-Fertilizied
Oil.
Figure 1. Comparison of the nC-18/phytane biodegradation index for different response treatments
on Arabian Crude oil in a Panicum sp. marsh mesocosm before (top) and after incubation (bottom) for six months. For all
treatments n=2. Key: OO-Oil Only, DO-Dispersed Oil, CO-Cleaned Oil, and FO-Fertilizied
Oil.
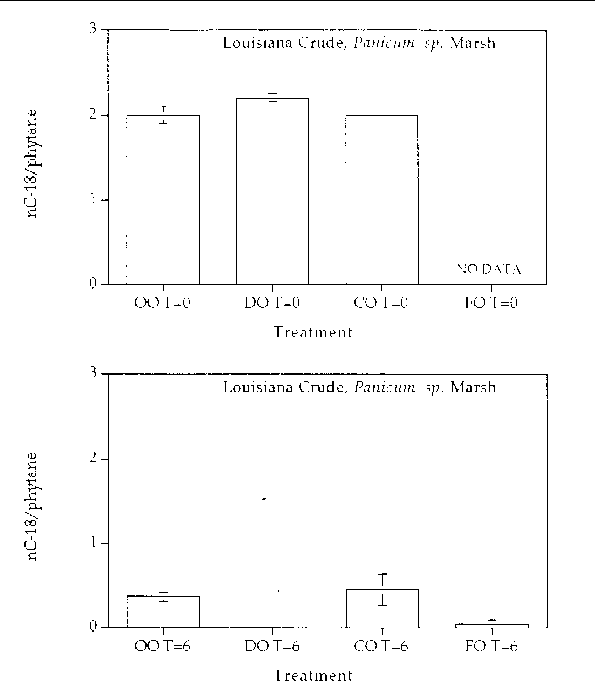 Figure 2. Comparison of the nC-18/phytane biodegradation index for different response treatments
on Louisiana Crude oil in a Panicum sp. marsh mesocosm before (top) and after incubation (bottom) for six months. For all
treatments n=2. Key: OO-Oil Only, DO-Dispersed Oil, CO-Cleaned Oil, and FO-Fertilizied
Oil.
Figure 2. Comparison of the nC-18/phytane biodegradation index for different response treatments
on Louisiana Crude oil in a Panicum sp. marsh mesocosm before (top) and after incubation (bottom) for six months. For all
treatments n=2. Key: OO-Oil Only, DO-Dispersed Oil, CO-Cleaned Oil, and FO-Fertilizied
Oil.
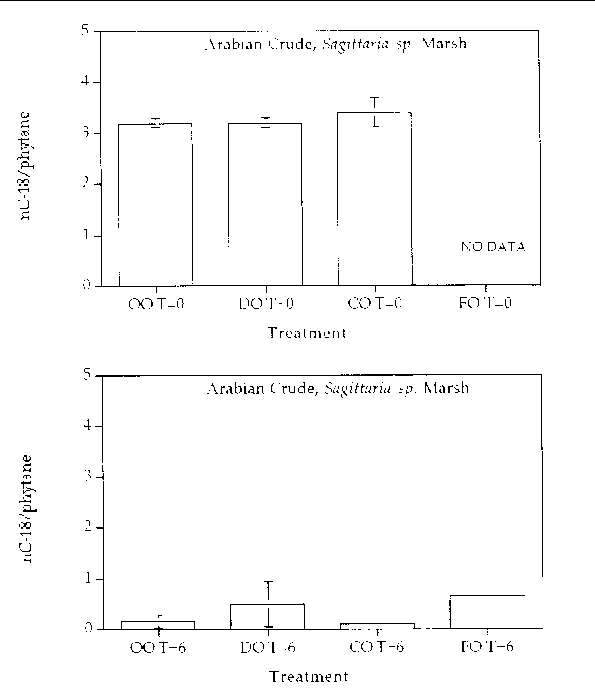 Figure 3. Comparison of the nC-18/phytane biodegradation index for different response treatments
on Arabian Crude oil in a Sagittaria sp. marsh mesocosm before (top) and after incubation (bottom) for six months. For all
treatments n=2. Key: OO-Oil Only, DO-Dispersed Oil, CO-Cleaned Oil, and FO-Fertilizied
Oil.
Figure 3. Comparison of the nC-18/phytane biodegradation index for different response treatments
on Arabian Crude oil in a Sagittaria sp. marsh mesocosm before (top) and after incubation (bottom) for six months. For all
treatments n=2. Key: OO-Oil Only, DO-Dispersed Oil, CO-Cleaned Oil, and FO-Fertilizied
Oil.
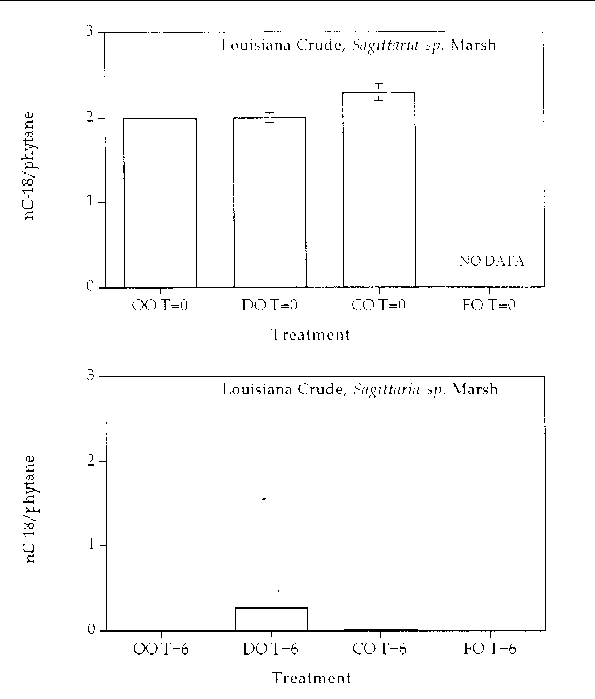 Figure 4. Comparison of the nC-18/phytane biodegradation index for different response treatments
on Louisiana Crude oil in a Sagittaria sp. marsh mesocosm before (top) and after incubation (bottom) for six months. For all
treatments n=2. Key: OO-Oil Only, DO-Dispersed Oil, CO-Cleaned Oil, and FO-Fertilizied
Oil.
Figure 4. Comparison of the nC-18/phytane biodegradation index for different response treatments
on Louisiana Crude oil in a Sagittaria sp. marsh mesocosm before (top) and after incubation (bottom) for six months. For all
treatments n=2. Key: OO-Oil Only, DO-Dispersed Oil, CO-Cleaned Oil, and FO-Fertilizied
Oil.
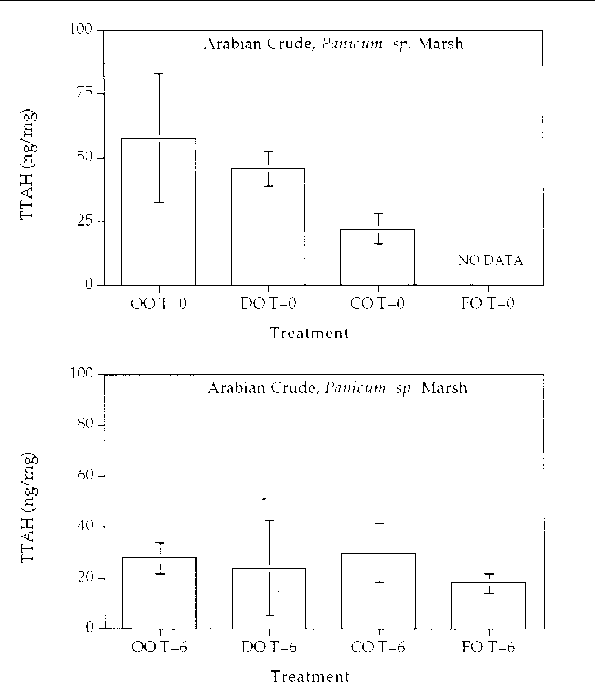 Figure 5. Comparison of the TTAH concentration for different response treatments on Arabian
Crude oil in a Panicum sp. marsh mesocosm before (top) and after incubation (bottom) for six months. For all
treatments n=2. Key: OO-Oil Only, DO-Dispersed Oil, CO-Cleaned Oil, and FO-Fertilizied
Oil.
Figure 5. Comparison of the TTAH concentration for different response treatments on Arabian
Crude oil in a Panicum sp. marsh mesocosm before (top) and after incubation (bottom) for six months. For all
treatments n=2. Key: OO-Oil Only, DO-Dispersed Oil, CO-Cleaned Oil, and FO-Fertilizied
Oil.
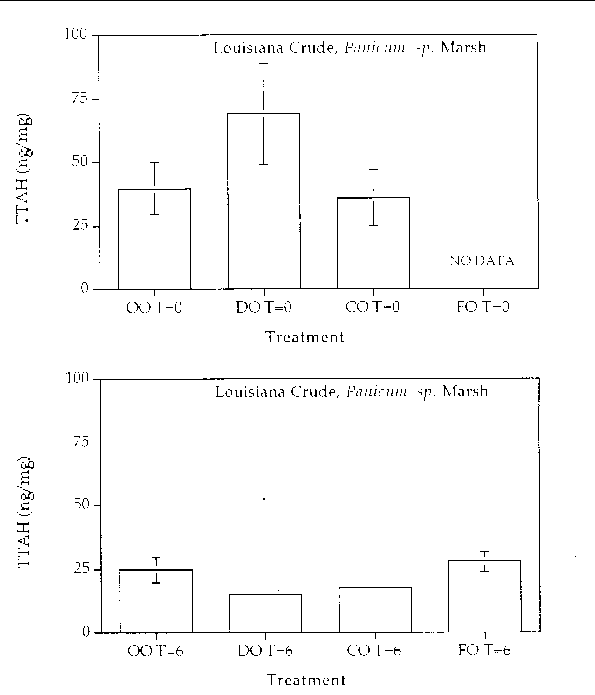 Figure 6. Comparison of the TTAH concentration for different response treatments on Louisiana
Crude oil in a Panicum sp. marsh mesocosm before (top) and after incubation (bottom) for six months. For all
treatments n=2. Key: OO-Oil Only, DO-Dispersed Oil, CO-Cleaned Oil, and FO-Fertilizied
Oil.
Figure 6. Comparison of the TTAH concentration for different response treatments on Louisiana
Crude oil in a Panicum sp. marsh mesocosm before (top) and after incubation (bottom) for six months. For all
treatments n=2. Key: OO-Oil Only, DO-Dispersed Oil, CO-Cleaned Oil, and FO-Fertilizied
Oil.
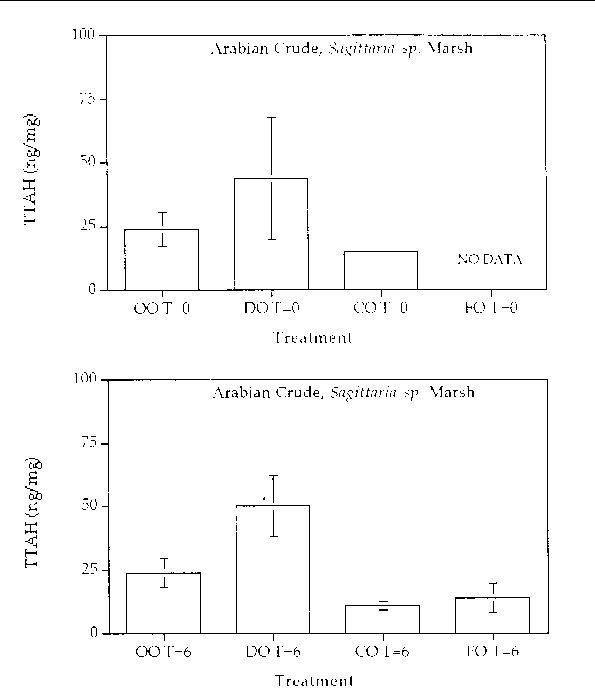 Figure 7. Comparison of the TTAH concentration for different response treatments on Arabian
Crude oil in a Sagittaria sp. marsh mesocosm before (top) and after incubation (bottom) for six months. For all
treatments n=2. Key: OO-Oil Only, DO-Dispersed Oil, CO-Cleaned Oil, and FO-Fertilizied
Oil.
Figure 7. Comparison of the TTAH concentration for different response treatments on Arabian
Crude oil in a Sagittaria sp. marsh mesocosm before (top) and after incubation (bottom) for six months. For all
treatments n=2. Key: OO-Oil Only, DO-Dispersed Oil, CO-Cleaned Oil, and FO-Fertilizied
Oil.
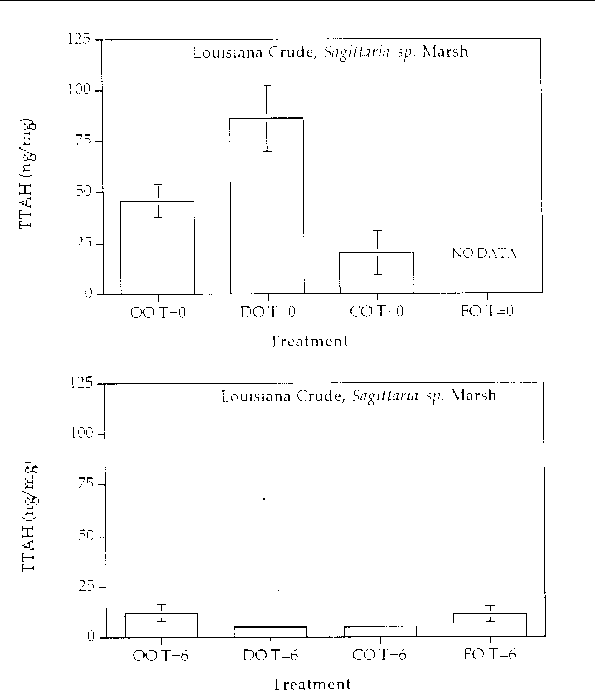 Figure 8. Comparison of the TTAH concentration for different response treatments on Louisiana
Crude oil in a Sagittaria sp. marsh mesocosm before (top) and after incubation (bottom) for six months. For all
treatments n=2. Key: OO-Oil Only, DO-Dispersed Oil, CO-Cleaned Oil, and FO-Fertilizied
Oil.
Figure 8. Comparison of the TTAH concentration for different response treatments on Louisiana
Crude oil in a Sagittaria sp. marsh mesocosm before (top) and after incubation (bottom) for six months. For all
treatments n=2. Key: OO-Oil Only, DO-Dispersed Oil, CO-Cleaned Oil, and FO-Fertilizied
Oil.
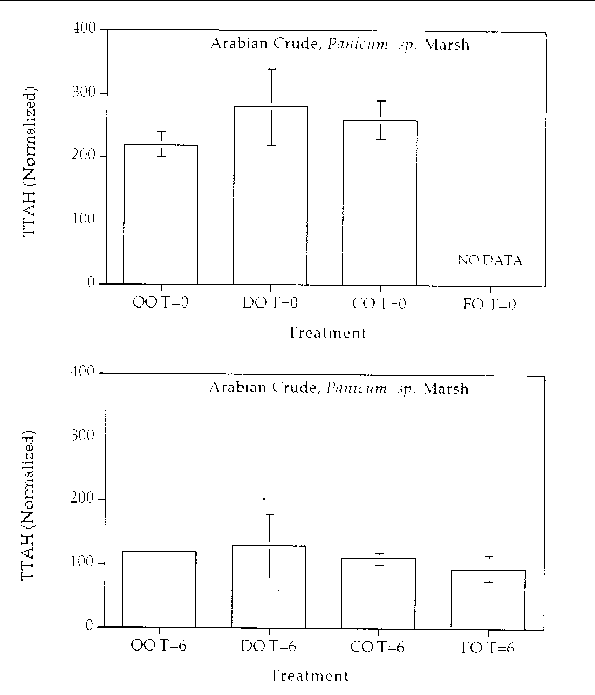 Figure 9. Comparison of the TTAH concentration normalized to the C-2 chrysenes for different
response treatments on Arabian Crude oil in a Panicum sp. marsh mesocosm before (top) and after incubation (bottom) for six months. For all
treatments n=2. Key: OO-Oil Only, DO-Dispersed Oil, CO-Cleaned Oil, and FO-Fertilizied
Oil.
Figure 9. Comparison of the TTAH concentration normalized to the C-2 chrysenes for different
response treatments on Arabian Crude oil in a Panicum sp. marsh mesocosm before (top) and after incubation (bottom) for six months. For all
treatments n=2. Key: OO-Oil Only, DO-Dispersed Oil, CO-Cleaned Oil, and FO-Fertilizied
Oil.
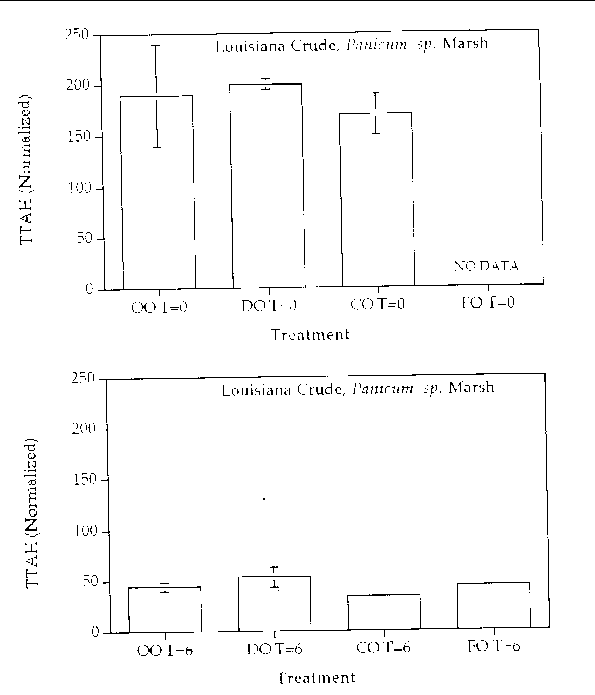 Figure 10. Comparison of the TTAH concentration normalized to the C-2 chrysenes for different
response treatments on Louisiana Crude oil in a Panicum sp. marsh mesocosm before (top) and after incubation (bottom) for six months. For all
treatments n=2. Key: OO-Oil Only, DO-Dispersed Oil, CO-Cleaned Oil, and FO-Fertilizied
Oil.
Figure 10. Comparison of the TTAH concentration normalized to the C-2 chrysenes for different
response treatments on Louisiana Crude oil in a Panicum sp. marsh mesocosm before (top) and after incubation (bottom) for six months. For all
treatments n=2. Key: OO-Oil Only, DO-Dispersed Oil, CO-Cleaned Oil, and FO-Fertilizied
Oil.
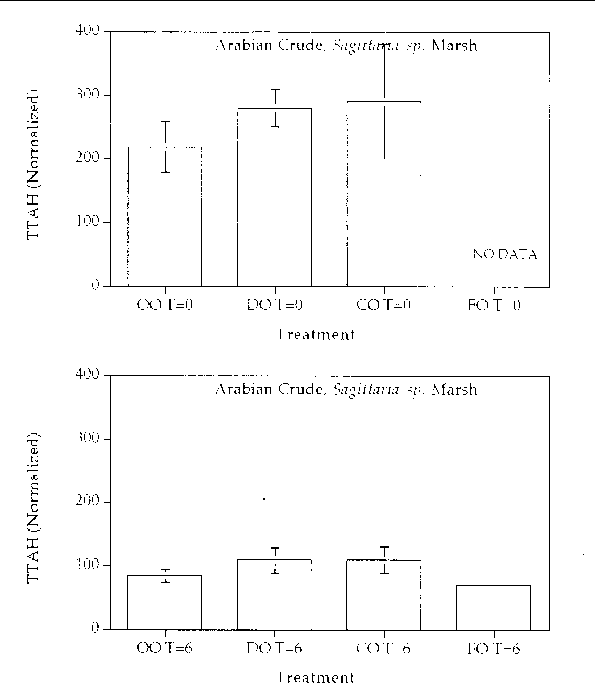 Figure 11. Comparison of the TTAH concentration normalized to the C-2 chrysenes for different
response treatments on Arabian Crude oil in a Sagittaria sp. marsh mesocosm before (top) and after incubation (bottom) for six months. For all
treatments n=2. Key: OO-Oil Only, DO-Dispersed Oil, CO-Cleaned Oil, and FO-Fertilizied
Oil.
Figure 11. Comparison of the TTAH concentration normalized to the C-2 chrysenes for different
response treatments on Arabian Crude oil in a Sagittaria sp. marsh mesocosm before (top) and after incubation (bottom) for six months. For all
treatments n=2. Key: OO-Oil Only, DO-Dispersed Oil, CO-Cleaned Oil, and FO-Fertilizied
Oil.
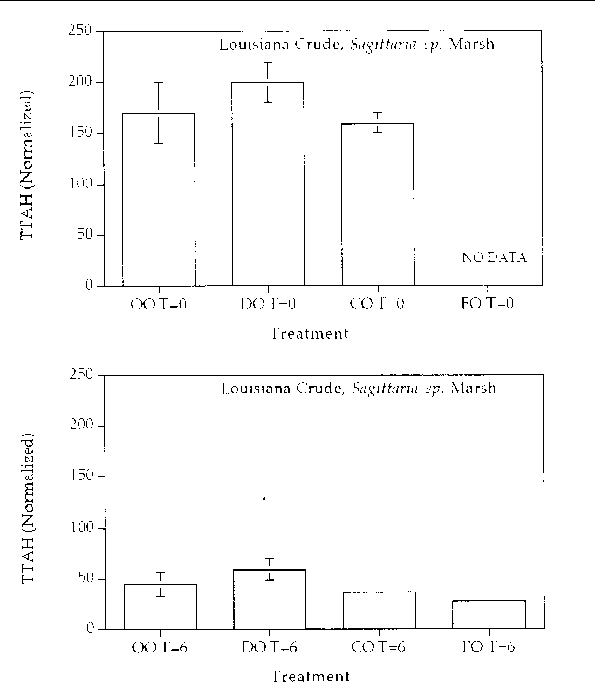 Figure 12. Comparison of the TTAH concentration normalized to the C-2 chrysenes for different
response treatments on Louisiana Crude oil in a Sagittaria sp. marsh mesocosm before (top) and after incubation (bottom) for six months. For all
treatments n=2. Key: OO-Oil Only, DO-Dispersed Oil, CO-Cleaned Oil, and FO-Fertilizied
Oil.
Figure 12. Comparison of the TTAH concentration normalized to the C-2 chrysenes for different
response treatments on Louisiana Crude oil in a Sagittaria sp. marsh mesocosm before (top) and after incubation (bottom) for six months. For all
treatments n=2. Key: OO-Oil Only, DO-Dispersed Oil, CO-Cleaned Oil, and FO-Fertilizied
Oil.
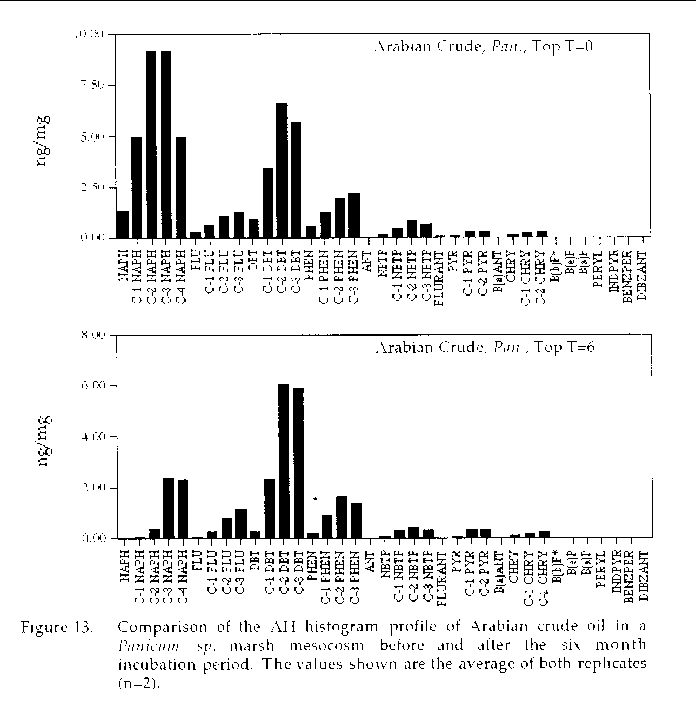 Figure 13. Comparison of the AH histogram profile of Arabian Crude oil in a Panicum sp. marsh mesocosm before and after the six month incubation period. The values shown
are the average of both replicates (n=2).
Figure 13. Comparison of the AH histogram profile of Arabian Crude oil in a Panicum sp. marsh mesocosm before and after the six month incubation period. The values shown
are the average of both replicates (n=2).
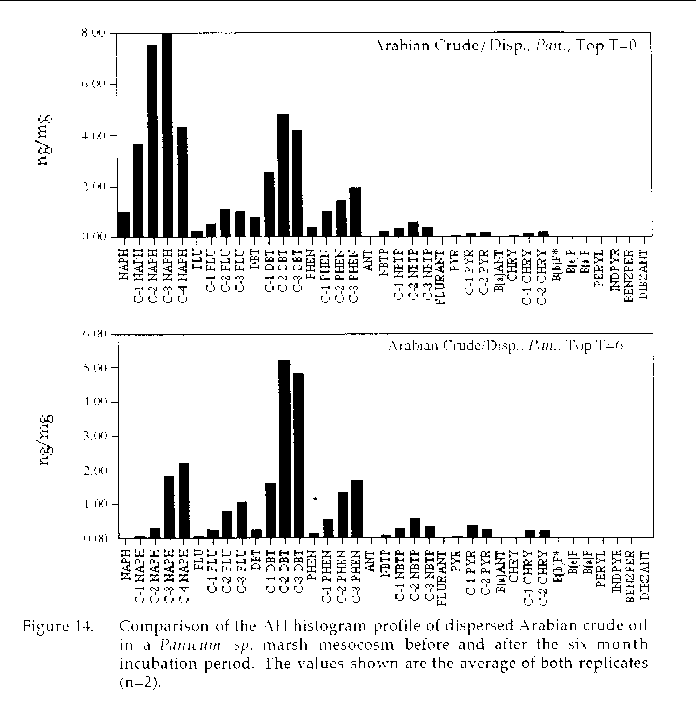 Figure 14. Comparison of the AH histogram profile of dispersed Arabian Crude oil in a Panicum sp. marsh mesocosm before and after the six month incubation period. The values shown
are the average of both replicates (n=2).
Figure 14. Comparison of the AH histogram profile of dispersed Arabian Crude oil in a Panicum sp. marsh mesocosm before and after the six month incubation period. The values shown
are the average of both replicates (n=2).
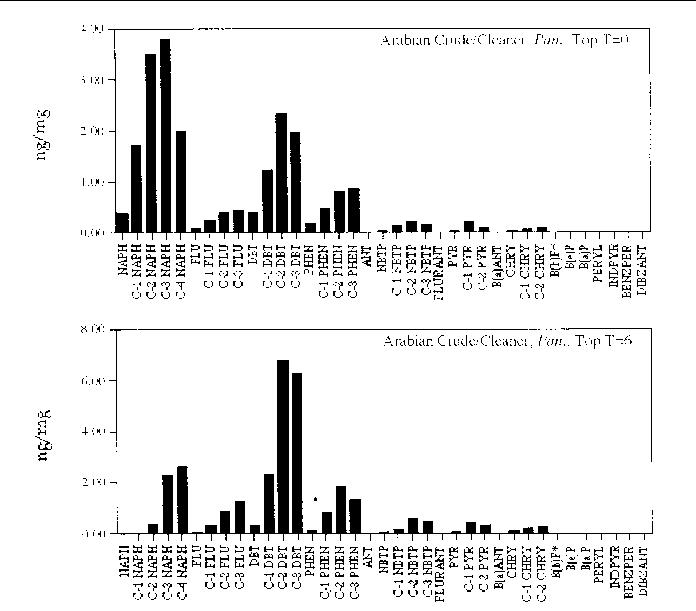 Figure 15. Comparison of the AH histogram profile of Arabian Crude oil mixed with a beach cleaner
in a Panicum sp. marsh mesocosm before and after the six month incubation period. The values shown
are the average of both replicates (n=2).
Figure 15. Comparison of the AH histogram profile of Arabian Crude oil mixed with a beach cleaner
in a Panicum sp. marsh mesocosm before and after the six month incubation period. The values shown
are the average of both replicates (n=2).
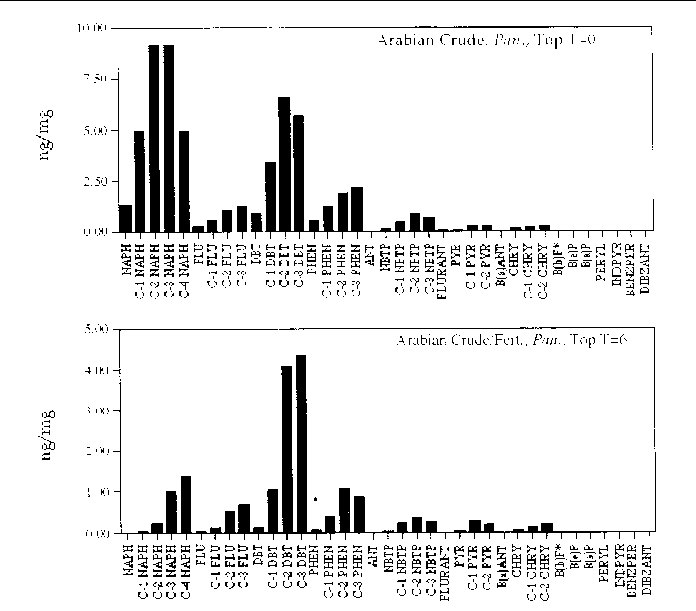 Figure 16. Comparison of the AH histogram profile of Arabian Crude oil with fertilizer in a
Panicum sp. marsh mesocosm before and after the six month incubation period. The values are the
average of both replicates (n=2).
Figure 16. Comparison of the AH histogram profile of Arabian Crude oil with fertilizer in a
Panicum sp. marsh mesocosm before and after the six month incubation period. The values are the
average of both replicates (n=2).
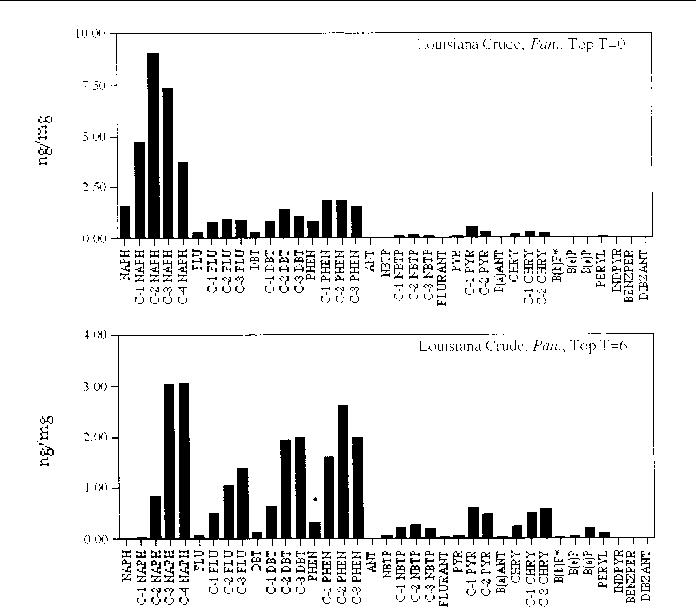 Figure 17. Comparison of the AH histogram profile of Louisiana Crude oil in a Panicum sp. marsh mesocosm before and after the six month incubation period. The values shown
are the average of both replicates (n=2).
Figure 17. Comparison of the AH histogram profile of Louisiana Crude oil in a Panicum sp. marsh mesocosm before and after the six month incubation period. The values shown
are the average of both replicates (n=2).
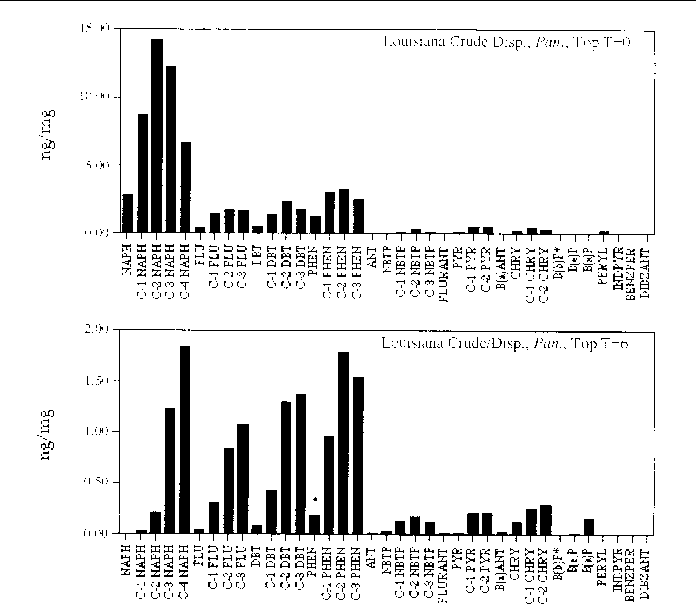 Figure 18. Comparison of the AH histogram profile of dispersed Louisiana Crude oil in a Panicum sp. marsh mesocosm before and after the six month incubation period. The values shown
are the average of both replicates (n=2).
Figure 18. Comparison of the AH histogram profile of dispersed Louisiana Crude oil in a Panicum sp. marsh mesocosm before and after the six month incubation period. The values shown
are the average of both replicates (n=2).
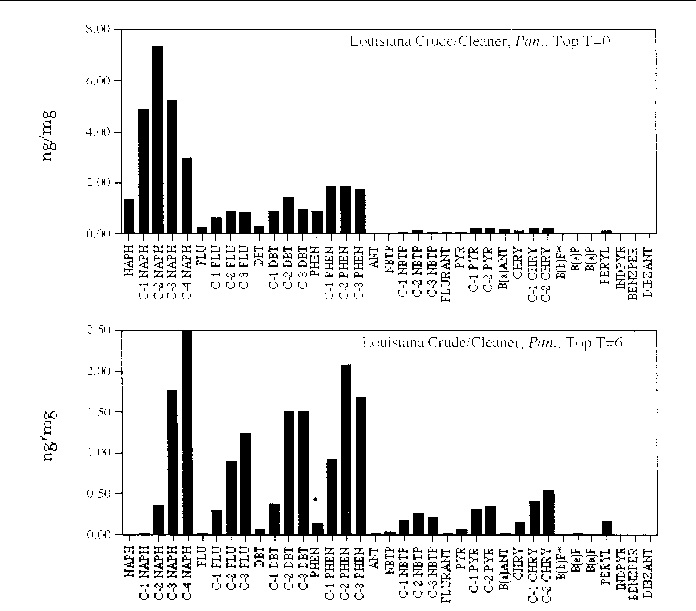 Figure 19. Comparison of the AH histogram profile of Louisiana Crude oil mixed with a beach
cleaner in a Panicum sp. marsh mesocosm before and after the six month incubation period. The values shown
are the average of both replicates (n=2).
Figure 19. Comparison of the AH histogram profile of Louisiana Crude oil mixed with a beach
cleaner in a Panicum sp. marsh mesocosm before and after the six month incubation period. The values shown
are the average of both replicates (n=2).
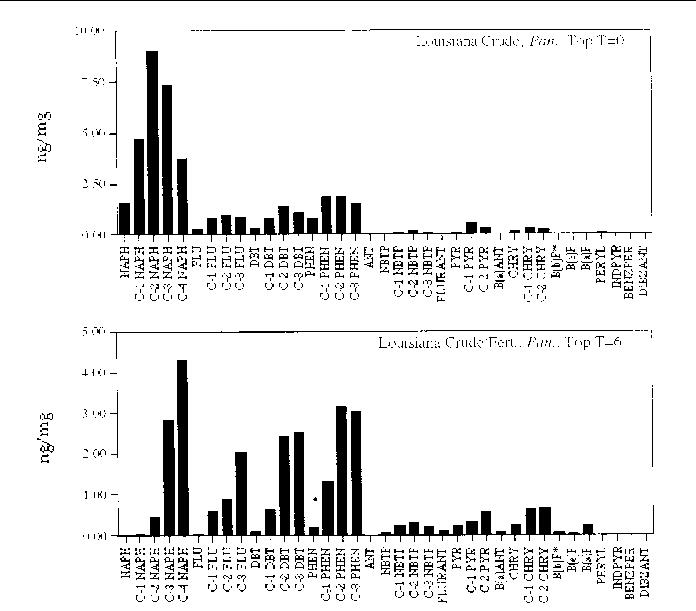 Figure 20. Comparison of the AH histogram profile of Louisiana Crude oil with additional fertilizer
in a Panicum sp. marsh mesocosm before and after the six month incubation period. The values shown
are the average of both replicates (n=2).
Figure 20. Comparison of the AH histogram profile of Louisiana Crude oil with additional fertilizer
in a Panicum sp. marsh mesocosm before and after the six month incubation period. The values shown
are the average of both replicates (n=2).
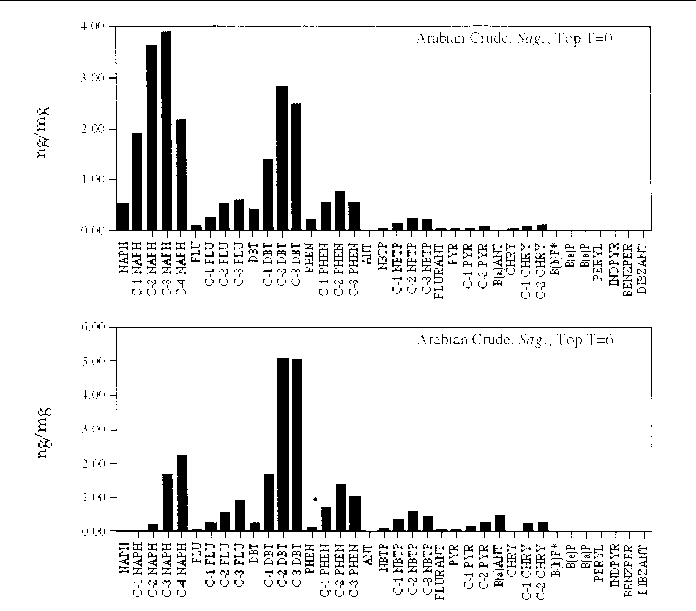 Figure 21. Comparison of the AH histogram profile of Arabian Crude oil in a Sagittaria sp. marsh mesocosm before and after the six month incubation period. The values shown
are the average of both replicates (n=2).
Figure 21. Comparison of the AH histogram profile of Arabian Crude oil in a Sagittaria sp. marsh mesocosm before and after the six month incubation period. The values shown
are the average of both replicates (n=2).
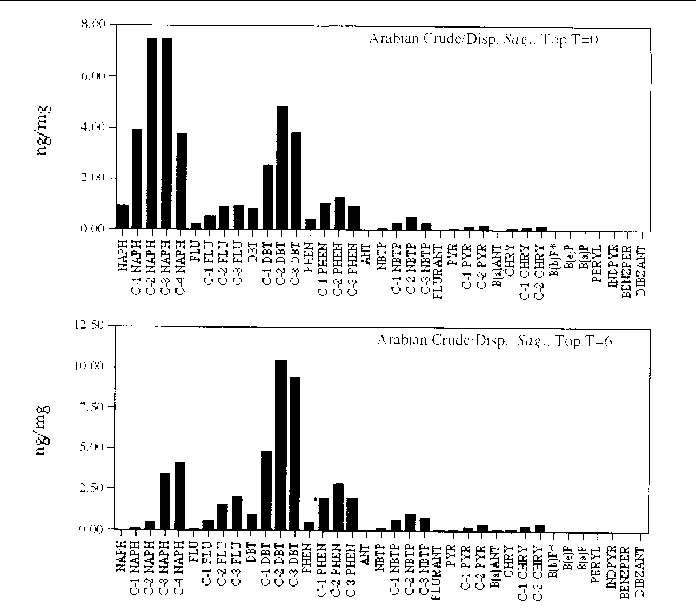 Figure 22. Comparison of the AH histogram profile of dispersed Arabian Crude oil in a Sagittaria sp. marsh mesocosm before and after the six month incubation period. The values shown
are the average of both replicates (n=2).
Figure 22. Comparison of the AH histogram profile of dispersed Arabian Crude oil in a Sagittaria sp. marsh mesocosm before and after the six month incubation period. The values shown
are the average of both replicates (n=2).
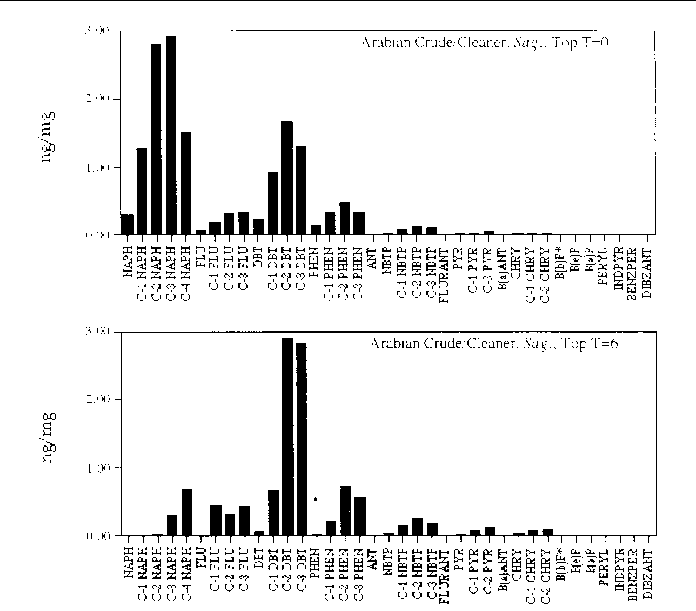 Figure 23. Comparison of the AH histogram profile of Arabian Crude oil mixed with a beach cleaner
in a Sagittaria sp. marsh mesocosm before and after the six month incubation period. The values shown
are the average of both replicates (n=2).
Figure 23. Comparison of the AH histogram profile of Arabian Crude oil mixed with a beach cleaner
in a Sagittaria sp. marsh mesocosm before and after the six month incubation period. The values shown
are the average of both replicates (n=2).
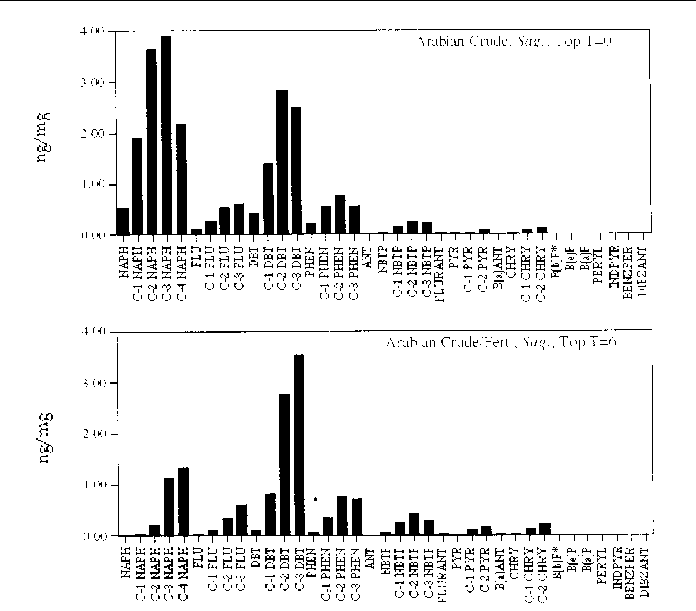 Figure 24. Comparison of the AH histogram profile of Arabian Crude oil with additional fertilizer
in a Sagittaria sp. marsh mesocosm before and after the six month incubation period. The values shown
are the average of both replicates (n=2).
Figure 24. Comparison of the AH histogram profile of Arabian Crude oil with additional fertilizer
in a Sagittaria sp. marsh mesocosm before and after the six month incubation period. The values shown
are the average of both replicates (n=2).
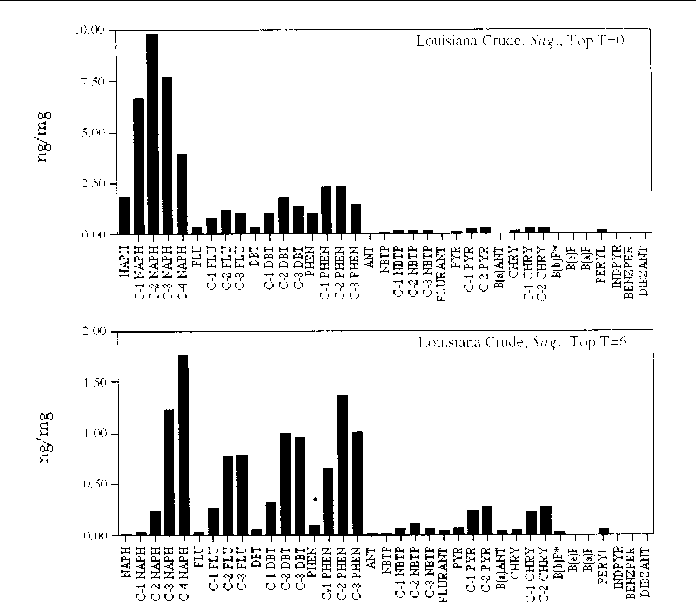 Figure 25. Comparison of the AH histogram profile of Louisiana Crude oil in a Sagittaria sp. marsh mesocosm before and after the six month incubation period. The values shown
are the average of both replicates (n=2).
Figure 25. Comparison of the AH histogram profile of Louisiana Crude oil in a Sagittaria sp. marsh mesocosm before and after the six month incubation period. The values shown
are the average of both replicates (n=2).
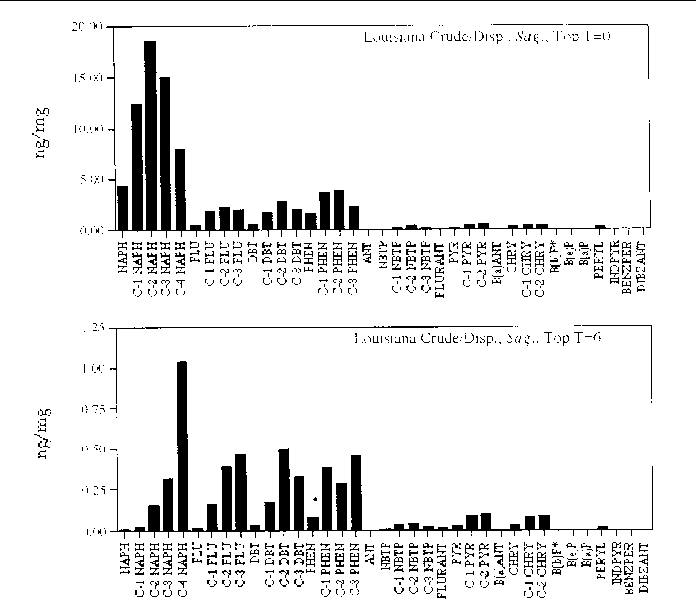 Figure 26. Comparison of the AH histogram profile of dispersed Louisiana Crude oil in a Sagittaria sp. marsh mesocosm before and after the six month incubation period. The values shown
are the average of both replicates (n=2).
Figure 26. Comparison of the AH histogram profile of dispersed Louisiana Crude oil in a Sagittaria sp. marsh mesocosm before and after the six month incubation period. The values shown
are the average of both replicates (n=2).
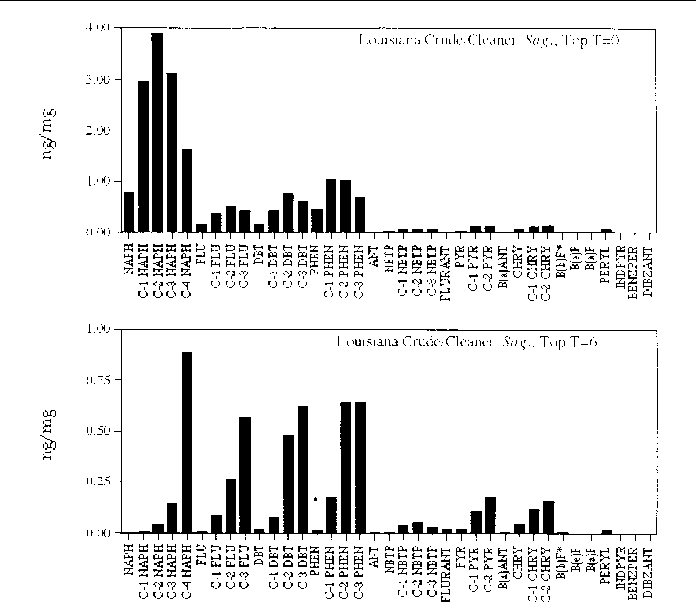 Figure 27. Comparison of the AH histogram profile of Louisiana Crude oil mixed with a beach
cleaner in a Sagittaria sp. marsh mseocosm before and after the six month incubation period. The values shown
are the average of both replicates (n=2).
Figure 27. Comparison of the AH histogram profile of Louisiana Crude oil mixed with a beach
cleaner in a Sagittaria sp. marsh mseocosm before and after the six month incubation period. The values shown
are the average of both replicates (n=2).
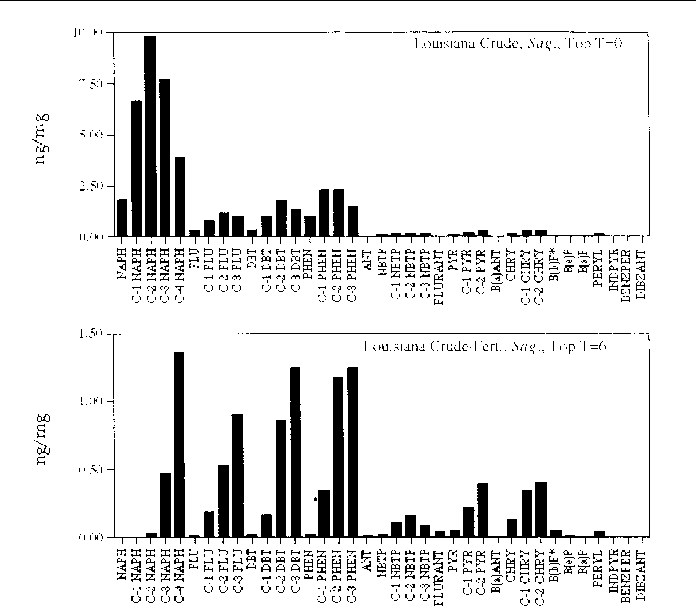 Figure 28. Comparison of the AH histogram profile of Louisiana Crude oil with additional fertilizer
in a Sagittaria sp. marsh mesocosm before and after the six month incubation period. The values shown
are the average of both replicates (n=2).
Figure 28. Comparison of the AH histogram profile of Louisiana Crude oil with additional fertilizer
in a Sagittaria sp. marsh mesocosm before and after the six month incubation period. The values shown
are the average of both replicates (n=2).
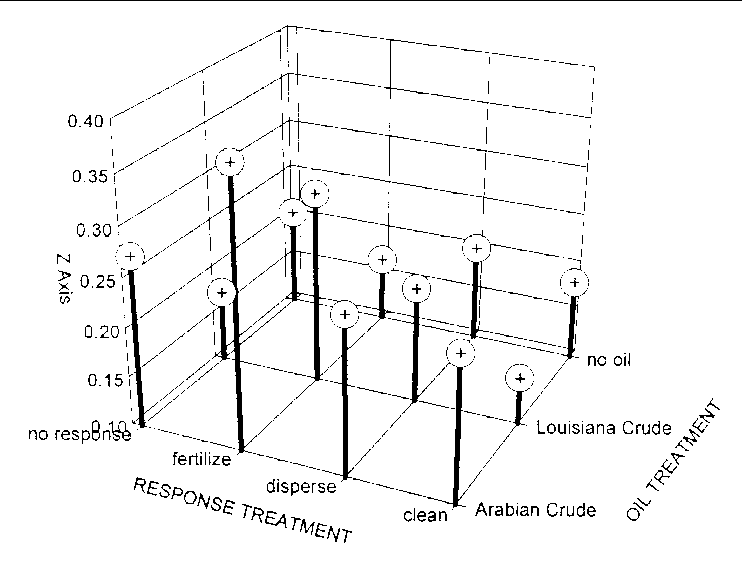 Figure 29. Average soil respiration rates (g C/hr) in the oil/response scenarios.
Figure 29. Average soil respiration rates (g C/hr) in the oil/response scenarios.
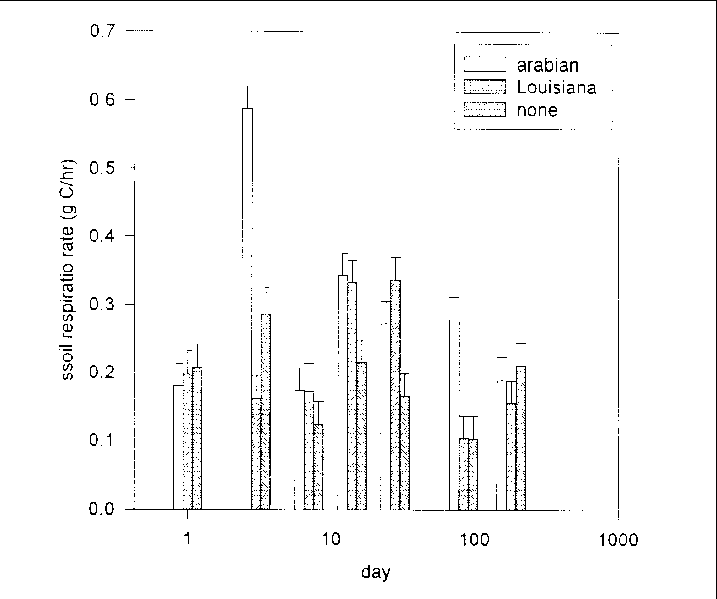 Figure 30. Soil respiration (g C/hr) among the different oil treatments over time.
Figure 30. Soil respiration (g C/hr) among the different oil treatments over time.
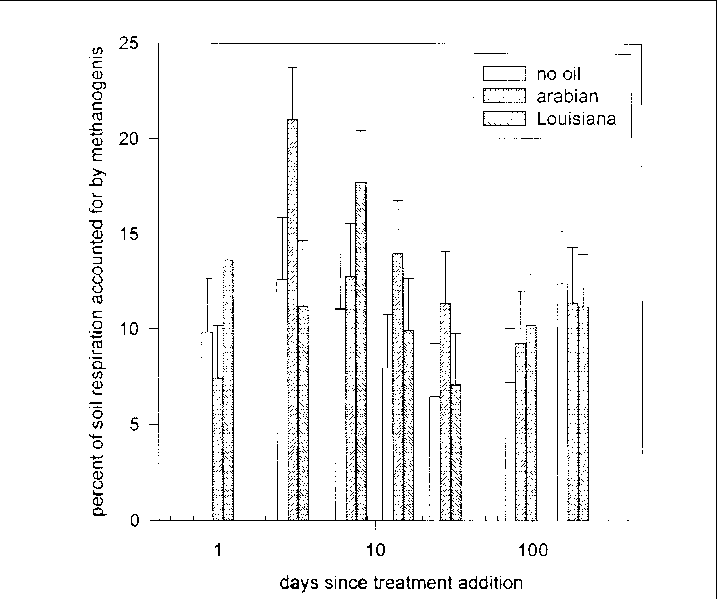 Figure 31. Percentage of respiration accounted for by methanogensis in the oil treatments.
Figure 31. Percentage of respiration accounted for by methanogensis in the oil treatments.
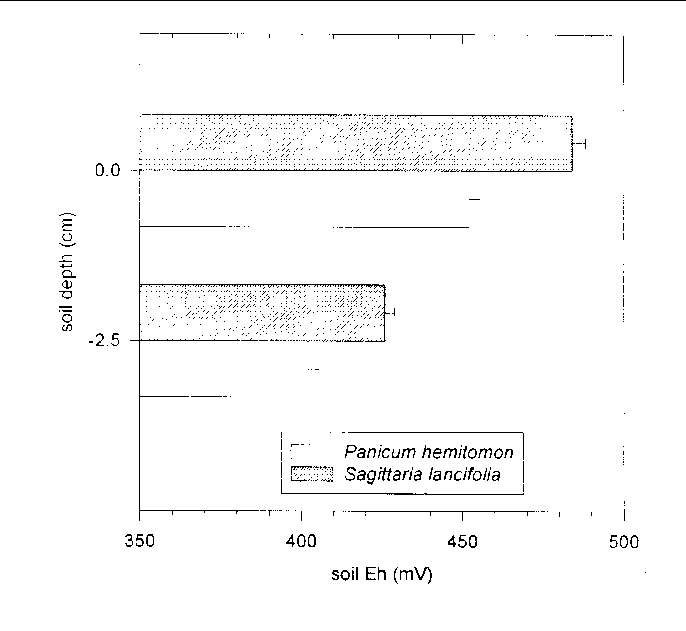 Figure 32. Soil Eh with depth in microcosms made using soil from Panicum hemitomon marsh and from Sagittaria lancifolia marsh.
Figure 32. Soil Eh with depth in microcosms made using soil from Panicum hemitomon marsh and from Sagittaria lancifolia marsh.
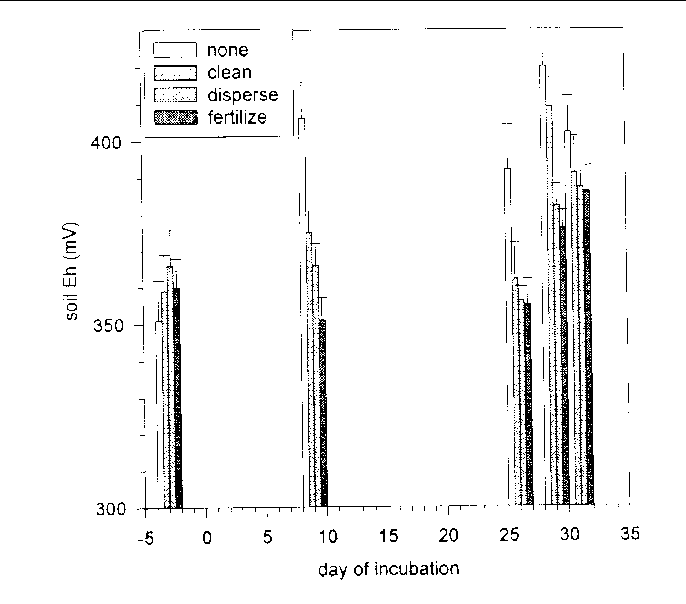 Figure 33. Soil Eh (mV) averaged over both depths over time among the response treatments.
Figure 33. Soil Eh (mV) averaged over both depths over time among the response treatments.
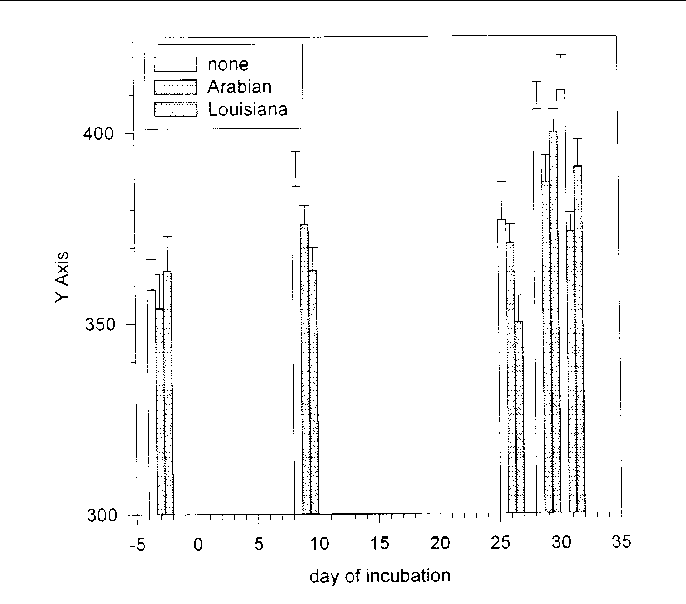 Figure 34. Soil Eh (mV) averaged over both depths over time among the oil treatments.
Figure 34. Soil Eh (mV) averaged over both depths over time among the oil treatments.